Common and distinct neural inputs to the medial central nucleus of the amygdala and anterior ventrolateral bed nucleus of stria terminalis in rats
- PMID: 22362201
- PMCID: PMC3662982
- DOI: 10.1007/s00429-012-0393-6
Common and distinct neural inputs to the medial central nucleus of the amygdala and anterior ventrolateral bed nucleus of stria terminalis in rats
Abstract
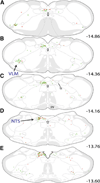
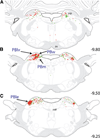
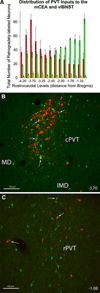
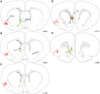
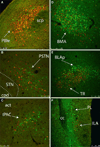
The central nucleus of the amygdala (CEA) and lateral bed nucleus of stria terminalis (BST) are highly interconnected limbic forebrain regions that share similar connectivity with other brain regions that coordinate behavioral and physiological responses to internal and environmental stressors. Their similar connectivity is frequently referred to when describing the CEA and lateral BST together as a unified "central extended amygdala". However, the CEA and BST reportedly play distinct roles in behavioral and physiological responses associated with fear, anxiety, and social defeat, presumably due to differences in connectivity. To identify common and unique sources of input to the CEA and lateral BST, we performed dual retrograde tracing. Fluorogold and cholera toxin β were iontophoresed into the medial CEA (CEAm) and the anterior ventrolateral BST (BSTvl) of adult male rats. The anatomical distribution of tracer-labeled neurons was mapped throughout the brain. Regions with overlapping populations of CEAm- and BSTvl-projecting neurons were further examined for the presence of double-labeled neurons. Although most regions with input to the mCEA also projected to the BSTvl, and vice versa, cortical and sensory system-related regions projected more robustly to the CEAm, while motor system-related regions primarily innervated the BSTvl. The incidence of double-labeled neurons with collateralized axonal inputs to the CEAm and BSTvl was relatively small (~2 to 13%) and varied across regions, suggesting regional differences in the degree of coordinated CEAm and BSTvl input. The demonstrated similarities and differences in inputs to CEAm and BSTvl provide new anatomical insights into the functional organization of these limbic forebrain regions.
Figures







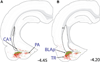


References
-
- Alheid G, Heimer L. New perspectives in basal forebrain organization of special relevance for neuropsychiatric disorders: the striatopallidal, amygdaloid, and corticopetal components of substantia innominata. Neuroscience. 1988;1:1–39. - PubMed
-
- Altschuler SM, Bao X, Bieger D, Hopkins DA, Miselis RR. Viscerotopic representation of the upper alimentary tract in the rat: sensory ganglia and nuclei of the solitary and spinal trigeminal tracts. J Comp Neurol. 1989;283(2):248–268. - PubMed
-
- Bhatnagar S, Dallman M. Neuroanatomical basis for facilitation of hypothalamic-pituitary-adrenal responses to a novel stressor after chronic stress. Neuroscience. 1998;84(4):1025–1039. - PubMed
-
- Bhatnagar S, Huber R, Nowak N, Trotter P. Lesions of the posterior paraventricular thalamus block habituation of hypothalamic-pituitary-adrenal responses to repeated restraint. J Neuroendocrinol. 2002;14(5):403–410. - PubMed
-
- Bhatnagar S, Huber R, Lazar E, Pych L, Vining C. Chronic stress alters behavior in the conditioned defensive burying test: role of the posterior paraventricular thalamus. Pharmacol Biochem Behav. 2003;76(2):343–349. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

