ROS constitute a convergence nexus in the development of IGF1 resistance and impaired wound healing in a rat model of type 2 diabetes
- PMID: 22362362
- PMCID: PMC3339831
- DOI: 10.1242/dmm.007872
ROS constitute a convergence nexus in the development of IGF1 resistance and impaired wound healing in a rat model of type 2 diabetes
Abstract
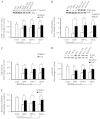
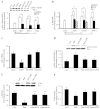
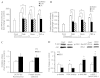
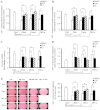
An indolent non-healing wound and insulin and/or insulin-like growth factor (IGF1) resistance are cardinal features of diabetes, inflammation and hypercortisolemia. Little is known about why these phenomena occur in so many contexts. Do the various triggers that induce insulin and/or IGF1 resistance and retard wound healing act through a common mechanism? Cultured dermal fibroblasts from rats and full-thickness excisional wounds were used as models to test the premise that reactive oxygen species (ROS) play a causal role in the development of IGF1 resistance and impaired wound healing under different but pathophysiologically relevant clinical settings, including diabetes, dexamethasone-induced hypercortisolemia and TNFα-induced inflammation. In normal fibroblasts, IGF1 initiated a strong degree of phosphorylation of insulin receptor substrate 1 (IRS1) (Tyr612) and Akt (Ser473), concomitantly with increased PI3K activity. This phenomenon seemed to be attenuated in fibroblasts that had phenotypic features of diabetes, inflammation or hypercortisolemia. Notably, these cells also exhibited an increase in the activity of the ROS-phospho-JNK (p-JNK)-p-IRS1 (Ser307) axis. The above-mentioned defects were reflected functionally by attenuation in IGF1-dependent stimulation of key fibroblast functions, including collagen synthesis and cell proliferation, migration and contraction. The effects of IGF1 on glucose disposal and cutaneous wound healing were also impaired in diabetic or hypercortisolemic rats. The ROS suppressors EUK-134 and α-lipoic acid, or small interfering RNA (siRNA)-mediated silencing of JNK expression, restored IGF1 sensitivity both in vitro and in vivo, and also ameliorated the impairment in IGF1-mediated wound responses during diabetes, inflammation and hypercortisolemia. Our data advance the notion that ROS constitute a convergence nexus for the development of IGF1 resistance and impaired wound healing under different but pathophysiologically relevant clinical settings, with a proof of concept for the beneficial effect of ROS suppressors.
Figures
Similar articles
-
NGF accelerates cutaneous wound healing by promoting the migration of dermal fibroblasts via the PI3K/Akt-Rac1-JNK and ERK pathways.Biomed Res Int. 2014;2014:547187. doi: 10.1155/2014/547187. Epub 2014 May 21. Biomed Res Int. 2014. PMID: 25006578 Free PMC article.
-
High Uric Acid Induces Insulin Resistance in Cardiomyocytes In Vitro and In Vivo.PLoS One. 2016 Feb 2;11(2):e0147737. doi: 10.1371/journal.pone.0147737. eCollection 2016. PLoS One. 2016. PMID: 26836389 Free PMC article.
-
The activation of the NF-κB-JNK pathway is independent of the PI3K-Rac1-JNK pathway involved in the bFGF-regulated human fibroblast cell migration.J Dermatol Sci. 2016 Apr;82(1):28-37. doi: 10.1016/j.jdermsci.2016.01.003. Epub 2016 Jan 7. J Dermatol Sci. 2016. PMID: 26829882
-
The role of the PI3K/AKT signalling pathway in the corneal epithelium: recent updates.Cell Death Dis. 2022 May 31;13(5):513. doi: 10.1038/s41419-022-04963-x. Cell Death Dis. 2022. PMID: 35641491 Free PMC article. Review.
-
Redox Signaling in Diabetic Wound Healing Regulates Extracellular Matrix Deposition.Antioxid Redox Signal. 2017 Oct 20;27(12):823-838. doi: 10.1089/ars.2017.7263. Epub 2017 Aug 10. Antioxid Redox Signal. 2017. PMID: 28699352 Free PMC article. Review.
Cited by
-
Comprehensive molecular characterization of microneedling therapy in a human three-dimensional skin model.PLoS One. 2018 Sep 20;13(9):e0204318. doi: 10.1371/journal.pone.0204318. eCollection 2018. PLoS One. 2018. PMID: 30235356 Free PMC article.
-
Antioxidant Properties of Plant-Derived Phenolic Compounds and Their Effect on Skin Fibroblast Cells.Antioxidants (Basel). 2021 May 5;10(5):726. doi: 10.3390/antiox10050726. Antioxidants (Basel). 2021. PMID: 34063059 Free PMC article. Review.
-
Targeting the Metabolic Paradigms in Cancer and Diabetes.Biomedicines. 2024 Jan 17;12(1):211. doi: 10.3390/biomedicines12010211. Biomedicines. 2024. PMID: 38255314 Free PMC article. Review.
-
Proanthocyanidins Prevent High Glucose-Induced Eye Malformation by Restoring Pax6 Expression in Chick Embryo.Nutrients. 2015 Aug 7;7(8):6567-81. doi: 10.3390/nu7085299. Nutrients. 2015. PMID: 26262640 Free PMC article.
-
The GSK-3β/Fyn/Nrf2 pathway in fibroblasts and wounds of type 2 diabetes: On the road to an evidence-based therapy of non-healing wounds.Adipocyte. 2012 Jul 1;1(3):161-163. doi: 10.4161/adip.20235. Adipocyte. 2012. PMID: 23700526 Free PMC article.
References
-
- Aguirre V., Uchida T., Yenush L., Davis R., White M. F. (2000). The c-Jun NH(2)-terminal kinase promotes insulin resistance during association with insulin receptor substrate-1 and phosphorylation of Ser(307). J. Biol. Chem. 275, 9047–9054 - PubMed
-
- Barja G. (2002). Rate of generation of oxidative stress-related damage and animal longevity. Free Radic. Biol. Med. 33, 1167–1172 - PubMed
-
- Beckert S., Haack S., Hierlemann H., Farrahi F., Mayer P., Konigsrainer A., Coerper S. (2007). Stimulation of steroid-suppressed cutaneous healing by repeated topical application of IGF-I: different mechanisms of action based upon the mode of IGF-I delivery. J. Surg. Res. 139, 217–221 - PubMed
-
- Bennett B. L., Satoh Y., Lewis A. J. (2003). JNK: a new therapeutic target for diabetes. Curr. Opin. Pharmacol. 3, 420–425 - PubMed
-
- Bitar M. S. (1997). Insulin-like growth factor-1 reverses diabetes-induced wound healing impairment in rats. Horm. Metab. Res. 29, 383–386 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

