Role of the lesion scar in the response to damage and repair of the central nervous system
- PMID: 22362507
- PMCID: PMC3375417
- DOI: 10.1007/s00441-012-1336-5
Role of the lesion scar in the response to damage and repair of the central nervous system
Abstract
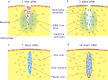
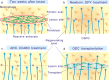
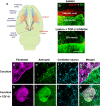
Traumatic damage to the central nervous system (CNS) destroys the blood-brain barrier (BBB) and provokes the invasion of hematogenous cells into the neural tissue. Invading leukocytes, macrophages and lymphocytes secrete various cytokines that induce an inflammatory reaction in the injured CNS and result in local neural degeneration, formation of a cystic cavity and activation of glial cells around the lesion site. As a consequence of these processes, two types of scarring tissue are formed in the lesion site. One is a glial scar that consists in reactive astrocytes, reactive microglia and glial precursor cells. The other is a fibrotic scar formed by fibroblasts, which have invaded the lesion site from adjacent meningeal and perivascular cells. At the interface, the reactive astrocytes and the fibroblasts interact to form an organized tissue, the glia limitans. The astrocytic reaction has a protective role by reconstituting the BBB, preventing neuronal degeneration and limiting the spread of damage. While much attention has been paid to the inhibitory effects of the astrocytic component of the scars on axon regeneration, this review will cover a number of recent studies in which manipulations of the fibroblastic component of the scar by reagents, such as blockers of collagen synthesis have been found to be beneficial for axon regeneration. To what extent these changes in the fibroblasts act via subsequent downstream actions on the astrocytes remains for future investigation.
Figures
Similar articles
-
Pharmacological Suppression of CNS Scarring by Deferoxamine Reduces Lesion Volume and Increases Regeneration in an In Vitro Model for Astroglial-Fibrotic Scarring and in Rat Spinal Cord Injury In Vivo.PLoS One. 2015 Jul 29;10(7):e0134371. doi: 10.1371/journal.pone.0134371. eCollection 2015. PLoS One. 2015. PMID: 26222542 Free PMC article.
-
Suppression of fibrotic scar formation promotes axonal regeneration without disturbing blood-brain barrier repair and withdrawal of leukocytes after traumatic brain injury.J Comp Neurol. 2010 Sep 15;518(18):3867-81. doi: 10.1002/cne.22431. J Comp Neurol. 2010. PMID: 20653039
-
Proliferating NG2-Cell-Dependent Angiogenesis and Scar Formation Alter Axon Growth and Functional Recovery After Spinal Cord Injury in Mice.J Neurosci. 2018 Feb 7;38(6):1366-1382. doi: 10.1523/JNEUROSCI.3953-16.2017. Epub 2017 Dec 26. J Neurosci. 2018. PMID: 29279310 Free PMC article.
-
The diversity and disparity of the glial scar.Nat Neurosci. 2018 Jan;21(1):9-15. doi: 10.1038/s41593-017-0033-9. Epub 2017 Dec 21. Nat Neurosci. 2018. PMID: 29269757 Free PMC article. Review.
-
Fibrotic scarring following lesions to the central nervous system.Matrix Biol. 2018 Aug;68-69:561-570. doi: 10.1016/j.matbio.2018.02.009. Epub 2018 Feb 9. Matrix Biol. 2018. PMID: 29428230 Review.
Cited by
-
Deficiency in matrix metalloproteinase-2 results in long-term vascular instability and regression in the injured mouse spinal cord.Exp Neurol. 2016 Oct;284(Pt A):50-62. doi: 10.1016/j.expneurol.2016.07.018. Epub 2016 Jul 25. Exp Neurol. 2016. PMID: 27468657 Free PMC article.
-
Positively Charged Oligo[Poly(Ethylene Glycol) Fumarate] Scaffold Implantation Results in a Permissive Lesion Environment after Spinal Cord Injury in Rat.Tissue Eng Part A. 2015 Jul;21(13-14):2099-114. doi: 10.1089/ten.TEA.2015.0019. Tissue Eng Part A. 2015. PMID: 25891264 Free PMC article.
-
Time course, distribution and cell types of induction of transforming growth factor betas following middle cerebral artery occlusion in the rat brain.PLoS One. 2012;7(10):e46731. doi: 10.1371/journal.pone.0046731. Epub 2012 Oct 8. PLoS One. 2012. PMID: 23056426 Free PMC article.
-
Effects of miR-219/miR-338 on microglia and astrocyte behaviors and astrocyte-oligodendrocyte precursor cell interactions.Neural Regen Res. 2020 Apr;15(4):739-747. doi: 10.4103/1673-5374.266922. Neural Regen Res. 2020. PMID: 31638099 Free PMC article.
-
Post-stroke remodeling processes in animal models and humans.J Cereb Blood Flow Metab. 2020 Jan;40(1):3-22. doi: 10.1177/0271678X19882788. Epub 2019 Oct 23. J Cereb Blood Flow Metab. 2020. PMID: 31645178 Free PMC article. Review.
References
-
- Abnet K, Fawcett JW, Dunnett SB. Interactions between meningeal cells and astrocytes in vivo and in vitro. Brain Res Dev Brain Res. 1991;59:187–196. - PubMed
-
- Alonso G, Privat A. Neuropeptide Y-producing neurons of the arcuate nucleus regenerate axons after surgical deafferentation of the mediobasal hypothalamus. J Neurosci Res. 1993;34:510–522. - PubMed
-
- Alonso G, Privat A. Reactive astrocytes involved in the formation of lesional scars differ in the mediobasal hypothalamus and in other forebrain regions. J Neurosci Res. 1993;34:523–538. - PubMed
-
- Asher RA, Morgenstern DA, Moon LD, Fawcett JW. Chondroitin sulphate proteoglycans: inhibitory components of the glial scar. Prog Brain Res. 2001;132:611–619. - PubMed
-
- Banker GA. Trophic interactions between astroglial cells and hippocampal neurons in culture. Science. 1980;209:809–810. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

