A bridging [4Fe-4S] cluster and nucleotide binding are essential for function of the Cfd1-Nbp35 complex as a scaffold in iron-sulfur protein maturation
- PMID: 22362766
- PMCID: PMC3320986
- DOI: 10.1074/jbc.M111.328914
A bridging [4Fe-4S] cluster and nucleotide binding are essential for function of the Cfd1-Nbp35 complex as a scaffold in iron-sulfur protein maturation
Abstract
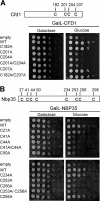
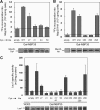
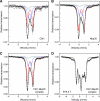
The essential P-loop NTPases Cfd1 and Nbp35 of the cytosolic iron-sulfur (Fe-S) protein assembly machinery perform a scaffold function for Fe-S cluster synthesis. Both proteins contain a nucleotide binding motif of unknown function and a C-terminal motif with four conserved cysteine residues. The latter motif defines the Mrp/Nbp35 subclass of P-loop NTPases and is suspected to be involved in transient Fe-S cluster binding. To elucidate the function of these two motifs, we first created cysteine mutant proteins of Cfd1 and Nbp35 and investigated the consequences of these mutations by genetic, cell biological, biochemical, and spectroscopic approaches. The two central cysteine residues (CPXC) of the C-terminal motif were found to be crucial for cell viability, protein function, coordination of a labile [4Fe-4S] cluster, and Cfd1-Nbp35 hetero-tetramer formation. Surprisingly, the two proximal cysteine residues were dispensable for all these functions, despite their strict evolutionary conservation. Several lines of evidence suggest that the C-terminal CPXC motifs of Cfd1-Nbp35 coordinate a bridging [4Fe-4S] cluster. Upon mutation of the nucleotide binding motifs Fe-S clusters could no longer be assembled on these proteins unless wild-type copies of Cfd1 and Nbp35 were present in trans. This result indicated that Fe-S cluster loading on these scaffold proteins is a nucleotide-dependent step. We propose that the bridging coordination of the C-terminal Fe-S cluster may be ideal for its facile assembly, labile binding, and efficient transfer to target Fe-S apoproteins, a step facilitated by the cytosolic iron-sulfur (Fe-S) protein assembly proteins Nar1 and Cia1 in vivo.
Figures






Similar articles
-
The Cfd1-Nbp35 complex acts as a scaffold for iron-sulfur protein assembly in the yeast cytosol.Nat Chem Biol. 2007 May;3(5):278-86. doi: 10.1038/nchembio872. Epub 2007 Apr 1. Nat Chem Biol. 2007. PMID: 17401378
-
The Yeast Nbp35-Cfd1 Cytosolic Iron-Sulfur Cluster Scaffold Is an ATPase.J Biol Chem. 2015 Sep 25;290(39):23793-802. doi: 10.1074/jbc.M115.667022. Epub 2015 Jul 20. J Biol Chem. 2015. PMID: 26195633 Free PMC article.
-
Function and crystal structure of the dimeric P-loop ATPase CFD1 coordinating an exposed [4Fe-4S] cluster for transfer to apoproteins.Proc Natl Acad Sci U S A. 2018 Sep 25;115(39):E9085-E9094. doi: 10.1073/pnas.1807762115. Epub 2018 Sep 10. Proc Natl Acad Sci U S A. 2018. PMID: 30201724 Free PMC article.
-
Mechanisms of Mitochondrial Iron-Sulfur Protein Biogenesis.Annu Rev Biochem. 2020 Jun 20;89:471-499. doi: 10.1146/annurev-biochem-013118-111540. Epub 2020 Jan 14. Annu Rev Biochem. 2020. PMID: 31935115 Review.
-
Fe-S Cluster Hsp70 Chaperones: The ATPase Cycle and Protein Interactions.Methods Enzymol. 2017;595:161-184. doi: 10.1016/bs.mie.2017.07.004. Epub 2017 Aug 21. Methods Enzymol. 2017. PMID: 28882200 Free PMC article. Review.
Cited by
-
Requirements of the cytosolic iron-sulfur cluster assembly pathway in Arabidopsis.Philos Trans R Soc Lond B Biol Sci. 2013 Jun 10;368(1622):20120259. doi: 10.1098/rstb.2012.0259. Print 2013 Jul 19. Philos Trans R Soc Lond B Biol Sci. 2013. PMID: 23754812 Free PMC article.
-
Unraveling the mechanism of [4Fe-4S] cluster assembly on the N-terminal cluster binding site of NUBP1.Protein Sci. 2023 May;32(5):e4625. doi: 10.1002/pro.4625. Protein Sci. 2023. PMID: 36916754 Free PMC article.
-
Cytosolic iron-sulfur protein assembly system identifies clients by a C-terminal tripeptide.Proc Natl Acad Sci U S A. 2023 Oct 31;120(44):e2311057120. doi: 10.1073/pnas.2311057120. Epub 2023 Oct 26. Proc Natl Acad Sci U S A. 2023. PMID: 37883440 Free PMC article.
-
Sulfur Administration in Fe-S Cluster Homeostasis.Antioxidants (Basel). 2021 Oct 29;10(11):1738. doi: 10.3390/antiox10111738. Antioxidants (Basel). 2021. PMID: 34829609 Free PMC article. Review.
-
AcsF Catalyzes the ATP-dependent Insertion of Nickel into the Ni,Ni-[4Fe4S] Cluster of Acetyl-CoA Synthase.J Biol Chem. 2016 Aug 26;291(35):18129-38. doi: 10.1074/jbc.M116.731638. Epub 2016 Jul 5. J Biol Chem. 2016. PMID: 27382049 Free PMC article.
References
-
- Beinert H. (2000) Iron-sulfur proteins. Ancient structures, still full of surprises. J. Biol. Inorg. Chem. 5, 2–15 - PubMed
-
- Lill R. (2009) Function and biogenesis iron-sulfur proteins. Nature 460, 831–838 - PubMed
-
- Johnson D. C., Dean D. R., Smith A. D., Johnson M. K. (2005) Structure, function, and formation of biological iron-sulfur clusters. Ann. Rev. Biochem. 74, 247–281 - PubMed
-
- Lill R., Mühlenhoff U. (2008) Maturation of iron-sulfur proteins in eukaryotes. Mechanisms, connected processes, and diseases. Annu. Rev. Biochem. 77, 669–700 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

