Upfront association of carboplatin plus pemetrexed in patients with brain metastases of lung adenocarcinoma
- PMID: 22362813
- PMCID: PMC3309855
- DOI: 10.1093/neuonc/nos004
Upfront association of carboplatin plus pemetrexed in patients with brain metastases of lung adenocarcinoma
Abstract
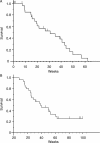
Approximately 10% of patients with non-small cell lung cancer (NSCLC) have brain metastases at the time of diagnosis. When surgical resection is not possible, whole brain radiotherapy is the standard of care, with a cerebral response rate of approximately 30%. We report our experience with an upfront association of carboplatin and pemetrexed (areas under the curve, 5 and 500 mg/m(2), respectively), every 3 weeks, in 30 patients presenting with newly diagnosed brain metastases and NSCLC. Cerebral MRIs were performed every 6-9 weeks. The radiologic response rates were assessed according to Response Evaluation Criteria in Solid Tumors. Overall survival was also determined. Twenty-six patients were evaluable for response, and the objective cerebral response rate (complete and partial response) in the intent-to-treat population was 40% (12 of 30 patients). Event-free survival was 31 weeks, and median overall survival was 39 weeks. The upfront association of carboplatin plus pemetrexed allows simultaneous treatment of cerebral and systemic disease in patients with NSCLC with newly diagnosed brain metastases and appears to be particularly interesting in terms of radiologic response and overall survival. Further clinical studies are warranted.
Figures
Similar articles
-
Pemetrexed and cisplatin combination with concurrent whole brain radiotherapy in patients with brain metastases of lung adenocarcinoma: a single-arm phase II clinical trial.J Neurooncol. 2013 May;112(3):461-6. doi: 10.1007/s11060-013-1079-5. Epub 2013 Feb 19. J Neurooncol. 2013. PMID: 23420398 Clinical Trial.
-
Randomized phase II and pharmacogenetic study of pemetrexed compared with pemetrexed plus carboplatin in pretreated patients with advanced non-small-cell lung cancer.J Clin Oncol. 2009 Apr 20;27(12):2038-45. doi: 10.1200/JCO.2008.19.1650. Epub 2009 Mar 23. J Clin Oncol. 2009. PMID: 19307503 Clinical Trial.
-
Pemetrexed and cisplatin as first-line chemotherapy for advanced non-small-cell lung cancer (NSCLC) with asymptomatic inoperable brain metastases: a multicenter phase II trial (GFPC 07-01).Ann Oncol. 2011 Nov;22(11):2466-2470. doi: 10.1093/annonc/mdr003. Epub 2011 Feb 14. Ann Oncol. 2011. PMID: 21321089 Clinical Trial.
-
Frontline Systemic Therapy With Pemetrexed-Platinum in Nonsquamous Non-Small-Cell Lung Cancer With Asymptomatic Brain Metastases.Am J Ther. 2017 Mar/Apr;24(2):e111-e120. doi: 10.1097/MJT.0000000000000106. Am J Ther. 2017. PMID: 25153672 Review.
-
Pemetrexed/cisplatin as first-line chemotherapy for advanced lung cancer with brain metastases: A case report and literature review.Medicine (Baltimore). 2016 Aug;95(32):e4401. doi: 10.1097/MD.0000000000004401. Medicine (Baltimore). 2016. PMID: 27512852 Free PMC article. Review.
Cited by
-
Icotinib as initial treatment in lung adenocarcinoma patients with brain metastases.Thorac Cancer. 2016 Jul;7(4):437-41. doi: 10.1111/1759-7714.12351. Epub 2016 Apr 5. Thorac Cancer. 2016. PMID: 27385986 Free PMC article.
-
Glutathione peroxidase 4-dependent glutathione high-consumption drives acquired platinum chemoresistance in lung cancer-derived brain metastasis.Clin Transl Med. 2021 Sep;11(9):e517. doi: 10.1002/ctm2.517. Clin Transl Med. 2021. PMID: 34586745 Free PMC article.
-
[Research progress of targeted therapy in non-small cell lung cancer brain metastases].Zhongguo Fei Ai Za Zhi. 2014 Nov;17(11):824-8. doi: 10.3779/j.issn.1009-3419.2014.11.09. Zhongguo Fei Ai Za Zhi. 2014. PMID: 25404274 Free PMC article. Review. Chinese.
-
Epidermal growth factor receptor tyrosine kinase inhibitors for the treatment of central nervous system metastases from non-small cell lung cancer: the present and the future.Transl Lung Cancer Res. 2016 Dec;5(6):563-578. doi: 10.21037/tlcr.2016.10.16. Transl Lung Cancer Res. 2016. PMID: 28149752 Free PMC article. Review.
-
Multicenter phase 2 study of patupilone for recurrent or progressive brain metastases from non-small cell lung cancer.Cancer. 2015 Dec 1;121(23):4165-72. doi: 10.1002/cncr.29636. Epub 2015 Aug 26. Cancer. 2015. PMID: 26308485 Free PMC article. Clinical Trial.
References
-
- Klos KJ, O'Neill BP. Brain metastases. Neurologist. 2004;10:31–46. doi:10.1097/01.nrl.0000106922.83090.71. - DOI - PubMed
-
- Posner JB. Neurologic complications of systemic cancer. Dis Mon. 1978;25:1–60. doi:10.1016/S0011-5029(78)80010-8. - DOI - PubMed
-
- Takakura K, Keji S, Shuntaro H, Asao H. Treatment. In: Takakura K, Sano K, Hojo S, editors. Metastatic Tumors of the Central Nervous System. Tokyo: Igaku Shoin; 1982. pp. 195–257.
-
- Sanchez de Cos J, Gonzalez MAS, Montero MV, et al. Non small cell lung cancer and silent brain metastasis survival and prognostic factors. Lung Cancer. 2009;63:140–145. doi:10.1016/j.lungcan.2008.04.013. - DOI - PubMed
-
- Patchell RA, Tibbs PA, Walsh JW, et al. A randomized trial of surgery in the treatment of single metastases to the brain. N Engl J Med. 1990;322:494–500. doi:10.1056/NEJM199002223220802. - DOI - PubMed