A revised architecture of primary cell walls based on biomechanical changes induced by substrate-specific endoglucanases
- PMID: 22362871
- PMCID: PMC3320196
- DOI: 10.1104/pp.111.192880
A revised architecture of primary cell walls based on biomechanical changes induced by substrate-specific endoglucanases
Abstract
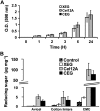
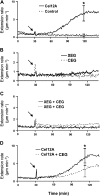
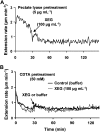
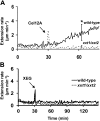
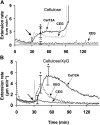
Xyloglucan is widely believed to function as a tether between cellulose microfibrils in the primary cell wall, limiting cell enlargement by restricting the ability of microfibrils to separate laterally. To test the biomechanical predictions of this "tethered network" model, we assessed the ability of cucumber (Cucumis sativus) hypocotyl walls to undergo creep (long-term, irreversible extension) in response to three family-12 endo-β-1,4-glucanases that can specifically hydrolyze xyloglucan, cellulose, or both. Xyloglucan-specific endoglucanase (XEG from Aspergillus aculeatus) failed to induce cell wall creep, whereas an endoglucanase that hydrolyzes both xyloglucan and cellulose (Cel12A from Hypocrea jecorina) induced a high creep rate. A cellulose-specific endoglucanase (CEG from Aspergillus niger) did not cause cell wall creep, either by itself or in combination with XEG. Tests with additional enzymes, including a family-5 endoglucanase, confirmed the conclusion that to cause creep, endoglucanases must cut both xyloglucan and cellulose. Similar results were obtained with measurements of elastic and plastic compliance. Both XEG and Cel12A hydrolyzed xyloglucan in intact walls, but Cel12A could hydrolyze a minor xyloglucan compartment recalcitrant to XEG digestion. Xyloglucan involvement in these enzyme responses was confirmed by experiments with Arabidopsis (Arabidopsis thaliana) hypocotyls, where Cel12A induced creep in wild-type but not in xyloglucan-deficient (xxt1/xxt2) walls. Our results are incompatible with the common depiction of xyloglucan as a load-bearing tether spanning the 20- to 40-nm spacing between cellulose microfibrils, but they do implicate a minor xyloglucan component in wall mechanics. The structurally important xyloglucan may be located in limited regions of tight contact between microfibrils.
Figures

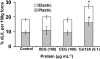


References
-
- Albersheim P, Darvill A, Roberts K, Sederoff R, Staehelin A. (2011) Plant Cell Walls, from Chemistry to Biology. Garland Science, New York, pp 227–272
-
- Baron-Epel O, Gharyal PK, Schindler M. (1988) Pectins as mediators of wall porosity in soybean cells. Planta 175: 389–395 - PubMed
-
- Bootten TJ, Harris PJ, Melton LD, Newman RH. (2004) Solid-state 13C-NMR spectroscopy shows that the xyloglucans in the primary cell walls of mung bean (Vigna radiata L.) occur in different domains: a new model for xyloglucan-cellulose interactions in the cell wall. J Exp Bot 55: 571–583 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

