Molecular mechanisms of acid-base sensing by the kidney
- PMID: 22362904
- PMCID: PMC3338302
- DOI: 10.1681/ASN.2012010029
Molecular mechanisms of acid-base sensing by the kidney
Abstract
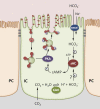
A major function of the kidney is to collaborate with the respiratory system to maintain systemic acid-base status within limits compatible with normal cell and organ function. It achieves this by regulating the excretion and recovery of bicarbonate (mainly in the proximal tubule) and the secretion of buffered protons (mainly in the distal tubule and collecting duct). How proximal tubular cells and distal professional proton transporting (intercalated) cells sense and respond to changes in pH, bicarbonate, and CO(2) status is a question that has intrigued many generations of renal physiologists. Over the past few years, however, some candidate molecular pH sensors have been identified, including acid/alkali-sensing receptors (GPR4, InsR-RR), kinases (Pyk2, ErbB1/2), pH-sensitive ion channels (ASICs, TASK, ROMK), and the bicarbonate-stimulated adenylyl cyclase (sAC). Some acid-sensing mechanisms in other tissues, such as CAII-PDK2L1 in taste buds, might also have similar roles to play in the kidney. Finally, the function of a variety of additional membrane channels and transporters is altered by pH variations both within and outside the cell, and the expression of several metabolic enzymes are altered by acid-base status in parts of the nephron. Thus, it is possible that a master pH sensor will never be identified. Rather, the kidney seems equipped with a battery of molecules that scan the epithelial cell environment to mount a coordinated physiologic response that maintains acid-base homeostasis. This review collates current knowledge on renal acid-base sensing in the context of a whole organ sensing and response process.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

