Hedgehog signalling regulates liver sinusoidal endothelial cell capillarisation
- PMID: 22362915
- PMCID: PMC3595101
- DOI: 10.1136/gutjnl-2011-301494
Hedgehog signalling regulates liver sinusoidal endothelial cell capillarisation
Abstract
Objective: Vascular remodelling during liver damage involves loss of healthy liver sinusoidal endothelial cell (LSEC) phenotype via capillarisation. Hedgehog (Hh) signalling regulates vascular development and increases during liver injury. This study therefore examined its role in capillarisation.
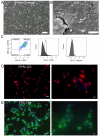
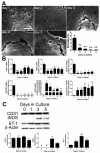
Design: Primary LSEC were cultured for 5 days to induce capillarisation. Pharmacological, antibody-mediated and genetic approaches were used to manipulate Hh signalling. Effects on mRNA and protein expression of Hh-regulated genes and capillarisation markers were evaluated by quantitative reverse transcription PCR and immunoblot. Changes in LSEC function were assessed by migration and tube forming assay, and gain/loss of fenestrae was examined by electron microscopy. Mice with acute or chronic liver injury were treated with Hh inhibitors; effects on capillarisation were assessed by immunohistochemistry.
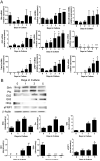
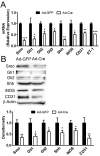
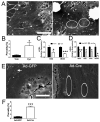
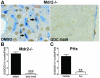
Results: Freshly isolated LSEC expressed Hh ligands, Hh receptors and Hh ligand antagonist Hhip. Capillarisation was accompanied by repression of Hhip and increased expression of Hh-regulated genes. Treatment with Hh agonist further induced expression of Hh ligands and Hh-regulated genes, and upregulated capillarisation-associated genes; whereas Hh signalling antagonist or Hh ligand neutralising antibody each repressed expression of Hh target genes and capillarisation markers. LSEC isolated from Smo(loxP/loxP) transgenic mice that had been infected with adenovirus expressing Cre-recombinase to delete Smoothened showed over 75% knockdown of Smoothened. During culture, Smoothened-deficient LSEC had inhibited Hh signalling, less induction of capillarisation-associated genes and retention of fenestrae. In mice with injured livers, inhibiting Hh signalling prevented capillarisation.
Conclusions: LSEC produce and respond to Hh ligands, and use Hh signalling to regulate complex phenotypic changes that occur during capillarisation.
Figures
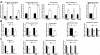


References
-
- Schaffner F, Poper H. Capillarisation of hepatic sinusoids in man. Gastroenterology. 1963;44:239–42. - PubMed
-
- Warren A, Bertolino P, Benseler V, et al. Marked changes of the hepatic sinusoid in a transgenic mouse model of acute immune-mediated hepatitis. J Hepatol. 2007;46:239–46. - PubMed
-
- Horn T, Junge J, Christoffersen P. Early alcoholic liver injury: changes of the Disse space in acinar zone 3. Liver. 1985;5:301–10. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
