The activities of current antimalarial drugs on the life cycle stages of Plasmodium: a comparative study with human and rodent parasites
- PMID: 22363211
- PMCID: PMC3283556
- DOI: 10.1371/journal.pmed.1001169
The activities of current antimalarial drugs on the life cycle stages of Plasmodium: a comparative study with human and rodent parasites
Abstract
Background: Malaria remains a disease of devastating global impact, killing more than 800,000 people every year-the vast majority being children under the age of 5. While effective therapies are available, if malaria is to be eradicated a broader range of small molecule therapeutics that are able to target the liver and the transmissible sexual stages are required. These new medicines are needed both to meet the challenge of malaria eradication and to circumvent resistance.
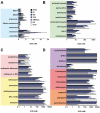
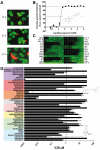
Methods and findings: Little is known about the wider stage-specific activities of current antimalarials that were primarily designed to alleviate symptoms of malaria in the blood stage. To overcome this critical gap, we developed assays to measure activity of antimalarials against all life stages of malaria parasites, using a diverse set of human and nonhuman parasite species, including male gamete production (exflagellation) in Plasmodium falciparum, ookinete development in P. berghei, oocyst development in P. berghei and P. falciparum, and the liver stage of P. yoelii. We then compared 50 current and experimental antimalarials in these assays. We show that endoperoxides such as OZ439, a stable synthetic molecule currently in clinical phase IIa trials, are strong inhibitors of gametocyte maturation/gamete formation and impact sporogony; lumefantrine impairs development in the vector; and NPC-1161B, a new 8-aminoquinoline, inhibits sporogony.
Conclusions: These data enable objective comparisons of the strengths and weaknesses of each chemical class at targeting each stage of the lifecycle. Noting that the activities of many compounds lie within achievable blood concentrations, these results offer an invaluable guide to decisions regarding which drugs to combine in the next-generation of antimalarial drugs. This study might reveal the potential of life-cycle-wide analyses of drugs for other pathogens with complex life cycles.
Conflict of interest statement
EW owns Novartis stock and receives compensation from Novartis. Some of the compounds which are contained in products sold by Novartis to treat malaria (e.g., coartem) are profiled herein. DL is employed by MMV but has no influence at all on the decision for funding a project. Indeed, such a decision is taken based on the recommendations of an independent External Scientific Advisory Committee. All other authors have declared that no competing interests exist.
Figures


References
-
- World Health Organization. World malaria report. 2010. Available: http://www.who.int/malaria/world_malaria_report_2010/en/. Accessed 6 December 2011.
-
- Medicines for Malaria Venture (MMV) MMV target product profiles. 2010. Available: http://www.mmv.org/research-development/essential-information-scientists.... Accessed 5 December 2011.
-
- Fidock DA. Drug discovery: priming the antimalarial pipeline. Nature. 2010;20:297–298. - PubMed
-
- Wells TNC, Alonso PL, Gutteridge WE. New medicines to improve control and contribute to the eradication of malaria. Nat Rev Drug Discov. 2009;8:879–891. - PubMed
-
- Sinden RE. A biologist's perspective on malaria vaccine development. Hum Vaccin. 2010;6:3–11. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

