Homocysteine and coronary heart disease: meta-analysis of MTHFR case-control studies, avoiding publication bias
- PMID: 22363213
- PMCID: PMC3283559
- DOI: 10.1371/journal.pmed.1001177
Homocysteine and coronary heart disease: meta-analysis of MTHFR case-control studies, avoiding publication bias
Abstract
Background: Moderately elevated blood levels of homocysteine are weakly correlated with coronary heart disease (CHD) risk, but causality remains uncertain. When folate levels are low, the TT genotype of the common C677T polymorphism (rs1801133) of the methylene tetrahydrofolate reductase gene (MTHFR) appreciably increases homocysteine levels, so "Mendelian randomization" studies using this variant as an instrumental variable could help test causality.
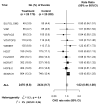
Methods and findings: Nineteen unpublished datasets were obtained (total 48,175 CHD cases and 67,961 controls) in which multiple genetic variants had been measured, including MTHFR C677T. These datasets did not include measurements of blood homocysteine, but homocysteine levels would be expected to be about 20% higher with TT than with CC genotype in the populations studied. In meta-analyses of these unpublished datasets, the case-control CHD odds ratio (OR) and 95% CI comparing TT versus CC homozygotes was 1.02 (0.98-1.07; p = 0.28) overall, and 1.01 (0.95-1.07) in unsupplemented low-folate populations. By contrast, in a slightly updated meta-analysis of the 86 published studies (28,617 CHD cases and 41,857 controls), the OR was 1.15 (1.09-1.21), significantly discrepant (p = 0.001) with the OR in the unpublished datasets. Within the meta-analysis of published studies, the OR was 1.12 (1.04-1.21) in the 14 larger studies (those with variance of log OR<0.05; total 13,119 cases) and 1.18 (1.09-1.28) in the 72 smaller ones (total 15,498 cases).
Conclusions: The CI for the overall result from large unpublished datasets shows lifelong moderate homocysteine elevation has little or no effect on CHD. The discrepant overall result from previously published studies reflects publication bias or methodological problems.
Conflict of interest statement
The Clinical Trial Service Unit has a policy of not accepting honoraria or other payments from the pharmaceutical industry, except for reimbursement of costs to participate in scientific meetings (RCl, DAB, SP, JCH, RCo, RP). PV and MDK are employees of Unilever R&D Vlaardingen, The Netherlands. Unilever makes no claims regarding B-vitamins, homocysteine, and CVD on their food products, and PV and MDK have worked on the paper due to their expertise and data from previous academic life. PV and MDK therefore do not consider this to be a competing interest but declare it for reasons of transparency. HH is an employee of deCode, a biotechnology company that produces genetic testing services. JD and RCo are on the Editorial Board of
Figures
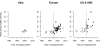



References
-
- Clarke R, Daly L, Robinson K, Naughton E, Cahalane S, et al. Hyperhomocysteinemia: an independent risk factor for vascular disease. N Engl J Med. 1991;324:1149–1155. - PubMed
-
- Homocysteine Studies Collaboration. Homocysteine and risk of ischemic heart disease and stroke: a meta-analysis. JAMA. 2002;288:2015–2022. - PubMed
-
- Clarke R, Halsey J, Lewington S, Lonn E, Armitage J, et al. Effects of lowering homocysteine levels with B vitamins on cardiovascular disease, cancer, and cause-specific mortality: Meta-analysis of 8 randomized trials involving 37 485 individuals. Arch Intern Med. 2010;170:1622–1631. - PubMed

