Natural products from the Lithistida: a review of the literature since 2000
- PMID: 22363244
- PMCID: PMC3280575
- DOI: 10.3390/md9122643
Natural products from the Lithistida: a review of the literature since 2000
Abstract
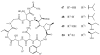
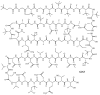
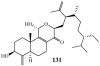
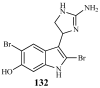
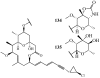
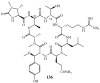
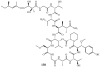
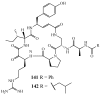
Lithistid sponges are known to produce a diverse array of compounds ranging from polyketides, cyclic and linear peptides, alkaloids, pigments, lipids, and sterols. A majority of these structurally complex compounds have very potent and interesting biological activities. It has been a decade since a thorough review has been published that summarizes the literature on the natural products reported from this amazing sponge order. This review provides an update on the current taxonomic classification of the Lithistida, describes structures and biological activities of 131 new natural products, and discusses highlights from the total syntheses of 16 compounds from marine sponges of the Order Lithistida providing a compilation of the literature since the last review published in 2002.
Keywords: Lithistida; Theonella; desmas; lithistid; natural product.
Figures



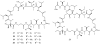

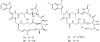

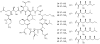

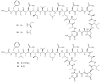

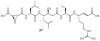
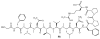



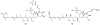
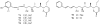
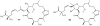
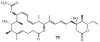

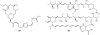
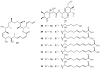


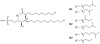

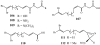

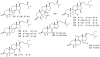
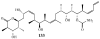
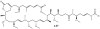

References
-
- Bewley C.A., Faulkner D.J. Lithistid sponges: Star performers or hosts to the stars. Angew. Chem. Int. Ed. 1998;37:2162–2178. - PubMed
-
- Hooper J.N.A., van Soest R.W.M. Systema Porifera: A Guide to the Classification of Sponges. Vol. 1 Kluwer Academic; New York, NY, USA: 2002.
-
- van Soest R.W.M., Boury-Esnault N., Hooper J.N.A., Rützler K., de Voogd N.J., Alvarez B., Hajdu E., Pisera A.B., Vacelet J., Manconi R., et al. World Porifera Database. [(accessed on 22 August 2011)]. Available online: http://www.marinespecies.org/porifera.
-
- Catalogue of Life: 2007 Annual Checklist: Species 2000 & ITIS Catalogue of Life Hierarchy. [(accessed on 18 August 2011)]. Available online: http://www.gbif.net.
-
- D’Auria M.V., Zampella A., Zollo F. The Chemistry of Lithistid Sponge: A Spectacular Source of New Metabolites. In: Atta-ur-Rahman, editor. Bioactive Natural Products. Part 7. Vol. 26. Elsevier; Amsterdam, The Netherlands: 2002. pp. 1175–1258.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

