Cyanotoxins: bioaccumulation and effects on aquatic animals
- PMID: 22363248
- PMCID: PMC3280578
- DOI: 10.3390/md9122729
Cyanotoxins: bioaccumulation and effects on aquatic animals
Abstract
Cyanobacteria are photosynthetic prokaryotes with wide geographic distribution that can produce secondary metabolites named cyanotoxins. These toxins can be classified into three main types according to their mechanism of action in vertebrates: hepatotoxins, dermatotoxins and neurotoxins. Many studies on the effects of cyanobacteria and their toxins over a wide range of aquatic organisms, including invertebrates and vertebrates, have reported acute effects (e.g., reduction in survivorship, feeding inhibition, paralysis), chronic effects (e.g., reduction in growth and fecundity), biochemical alterations (e.g., activity of phosphatases, GST, AChE, proteases), and behavioral alterations. Research has also focused on the potential for bioaccumulation and transferring of these toxins through the food chain. Although the herbivorous zooplankton is hypothesized as the main target of cyanotoxins, there is not unquestionable evidence of the deleterious effects of cyanobacteria and their toxins on these organisms. Also, the low toxin burden in secondary consumers points towards biodilution of microcystins in the food web as the predominant process. In this broad review we discuss important issues on bioaccumulation and the effects of cyanotoxins, with emphasis on microcystins, as well as drawbacks and future needs in this field of research.
Keywords: bioaccumulation; cyanobacteria; cyanotoxins; invertebrates; vertebrates.
Figures

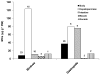
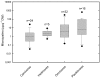
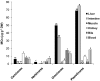
References
-
- Whitton B.A., Potts M. Introduction to the Cyanobacteria. In: Whitton B.A., Potts M., editors. The Ecology of Cyanobacteria, Their Diversity in Time and Space. 1st. Kluwer Academic Plublishers; Dordrecht, The Netherlands: 2000. pp. 1–4.
-
- Carmichael W.W. Cyanobacteria secondary metabolites: The cyanotoxins. J. Appl. Bacteriol. 1992;72:445–459. - PubMed
-
- Chorus I., Bartram J. In: Toxic Cyanobacteria in Water. A Guide to Their Public Health Consequences, Monitoring and Management. 1st. Chorus I., Bartran J., editors. E & FN Spon; London, UK: 1999.
-
- Codd G.A., Bell S.G., Kaya K., Ward C.J., Beattie K.A., Metcalf J.S. Cyanobacterial toxins, exposure routes and human health. Eur. J. Phycol. 1999;34:405–415.
-
- Sivonen K. Cyanobacterial toxins and toxin production. Phycologia. 1996;190:267–275.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

