Sexual differentiation of the rodent brain: dogma and beyond
- PMID: 22363256
- PMCID: PMC3282918
- DOI: 10.3389/fnins.2012.00026
Sexual differentiation of the rodent brain: dogma and beyond
Abstract
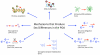
Steroid hormones of gonadal origin act on the neonatal brain to produce sex differences that underlie adult reproductive physiology and behavior. Neuronal sex differences occur on a variety of levels, including differences in regional volume and/or cell number, morphology, physiology, molecular signaling, and gene expression. In the rodent, many of these sex differences are determined by steroid hormones, particularly estradiol, and are established by diverse downstream effects. One brain region that is potently organized by estradiol is the preoptic area (POA), a region critically involved in many behaviors that show sex differences, including copulatory and maternal behaviors. This review focuses on the POA as a case study exemplifying the depth and breadth of our knowledge as well as the gaps in understanding the mechanisms through which gonadal hormones produce lasting neural and behavioral sex differences. In the POA, multiple cell types, including neurons, astrocytes, and microglia are masculinized by estradiol. Multiple downstream molecular mediators are involved, including prostaglandins, various glutamate receptors, protein kinase A, and several immune signaling molecules. Moreover, emerging evidence indicates epigenetic mechanisms maintain sex differences in the POA that are organized perinatally and thereby produce permanent behavioral changes. We also review emerging strategies to better elucidate the mechanisms through which genetics and epigenetics contribute to brain and behavioral sex differences.
Keywords: development; epigenetics; estradiol; hormone; preoptic area; sex difference.
Figures

References
-
- Adachi S., Fujioka H., Kakehashi C., Matsuwaki T., Nishihara M., Akema T. (2009). Possible involvement of microglia containing cyclooxygenase-1 in the accumulation of gonadotrophin-releasing hormone in the preoptic area in female rats. J. Neuroendocrinol. 21, 1029–103710.1111/j.1365-2826.2009.01928.x - DOI - PubMed
LinkOut - more resources
Full Text Sources

