Constitutive neutrophil apoptosis: regulation by cell concentration via S100 A8/9 and the MEK-ERK pathway
- PMID: 22363402
- PMCID: PMC3281816
- DOI: 10.1371/journal.pone.0029333
Constitutive neutrophil apoptosis: regulation by cell concentration via S100 A8/9 and the MEK-ERK pathway
Abstract
Programmed cell death (PCD) is a fundamental mechanism in tissue and cell homeostasis. It was long suggested that apoptosis regulates the cell number in diverse cell populations; however no clear mechanism was shown. Neutrophils are the short-lived, first-line defense of innate immunity, with an estimated t = 1/2 of 8 hours and a high turnover rate. Here we first show that spontaneous neutrophil constitutive PCD is regulated by cell concentrations. Using a proteomic approach, we identified the S100 A8/9 complex, which constitutes roughly 40% of cytosolic protein in neutrophils, as mediating this effect. We further demonstrate that it regulates cell survival via a signaling mechanism involving MEK-ERK via TLR4 and CD11B/CD18. This mechanism is suggested to have a fine-tuning role in regulating the neutrophil number in bone marrow, peripheral blood, and inflammatory sites.
Conflict of interest statement
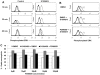
Figures





References
-
- Ellis RE, Yuan JY, Horvitz HR. Mechanisms and functions of cell death. Annu Rev Cell Biol. 1991;7:663–698. - PubMed
-
- Krammer PH. CD95′s deadly mission in the immune system. Nature. 2000;407:789–795. - PubMed
-
- Stark MA, Huo Y, Burcin TL, Morris MA, Olson TS, et al. Phagocytosis of apoptotic neutrophils regulates granulopoiesis via IL-23 and IL-17. Immunity. 2005;22:285–294. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

