A switch in hepatic cortisol metabolism across the spectrum of non alcoholic fatty liver disease
- PMID: 22363403
- PMCID: PMC3282715
- DOI: 10.1371/journal.pone.0029531
A switch in hepatic cortisol metabolism across the spectrum of non alcoholic fatty liver disease
Abstract
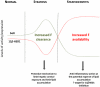
Context: Non alcoholic fatty liver disease (NAFLD) is the hepatic manifestation of the metabolic syndrome. NAFLD represents a spectrum of liver disease ranging from reversible hepatic steatosis, to non alcoholic steato-hepatitis (NASH) and cirrhosis. The potential role of glucocorticoids (GC) in the pathogenesis of NAFLD is highlighted in patients with GC excess, Cushing's syndrome, who develop central adiposity, insulin resistance and in 20% of cases, NAFLD. Although in most cases of NAFLD, circulating cortisol levels are normal, hepatic cortisol availability is controlled by enzymes that regenerate cortisol (F) from inactive cortisone (E) (11β-hydroxysteroid dehydrogenase type 1, 11β-HSD1), or inactivate cortisol through A-ring metabolism (5α- and 5β-reductase, 5αR and 5βR).
Objective and methods: In vitro studies defined 11β-HSD1 expression in normal and NASH liver samples. We then characterised hepatic cortisol metabolism in 16 patients with histologically proven NAFLD compared to 32 obese controls using gas chromatographic analysis of 24 hour urine collection and plasma cortisol generation profile following oral cortisone.
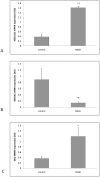
Results: In patients with steatosis 5αR activity was increased, with a decrease in hepatic 11β-HSD1 activity. Total cortisol metabolites were increased in this group consistent with increased GC production rate. In contrast, in patients with NASH, 11β-HSD1 activity was increased both in comparison to patients with steatosis, and controls. Endorsing these findings, 11β-HSD1 mRNA and immunostaining was markedly increased in NASH patients in peri septal hepatocytes and within CD68 positive macrophages within inflamed cirrhotic septa.
Conclusion: Patients with hepatic steatosis have increased clearance and decreased hepatic regeneration of cortisol and we propose that this may represent a protective mechanism to decrease local GC availability to preserve hepatic metabolic phenotype. With progression to NASH, increased 11β-HSD1 activity and consequent cortisol regeneration may serve to limit hepatic inflammation.
Conflict of interest statement
Figures



Similar articles
-
Hepatic 11beta-HSD1 mRNA expression in fatty liver and nonalcoholic steatohepatitis.Clin Endocrinol (Oxf). 2009 Apr;70(4):554-60. doi: 10.1111/j.1365-2265.2008.03358.x. Clin Endocrinol (Oxf). 2009. PMID: 18665910
-
Overexpression of hepatic 5α-reductase and 11β-hydroxysteroid dehydrogenase type 1 in visceral adipose tissue is associated with hyperinsulinemia in morbidly obese patients.Metabolism. 2011 Dec;60(12):1775-80. doi: 10.1016/j.metabol.2011.05.001. Epub 2011 Jun 24. Metabolism. 2011. PMID: 21704348
-
Lack of significant metabolic abnormalities in mice with liver-specific disruption of 11β-hydroxysteroid dehydrogenase type 1.Endocrinology. 2012 Jul;153(7):3236-48. doi: 10.1210/en.2012-1019. Epub 2012 May 3. Endocrinology. 2012. PMID: 22555437 Free PMC article.
-
Growth hormone, insulin-like growth factor-I and the cortisol-cortisone shuttle.Horm Res. 2001;56 Suppl 1:1-6. doi: 10.1159/000048126. Horm Res. 2001. PMID: 11786677 Review.
-
11beta-hydroxysteroid dehydrogenase type 1 and obesity.Front Horm Res. 2008;36:146-164. doi: 10.1159/000115363. Front Horm Res. 2008. PMID: 18230901 Review.
Cited by
-
Changes of androgen and corticosterone metabolites excretion and conversion in cystic fibrosis.Front Endocrinol (Lausanne). 2023 Aug 29;14:1244127. doi: 10.3389/fendo.2023.1244127. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 37711888 Free PMC article.
-
Steroid metabolomic signature of liver disease in nonsyndromic childhood obesity.Endocr Connect. 2019 Jun;8(6):764-771. doi: 10.1530/EC-18-0536. Endocr Connect. 2019. PMID: 31071683 Free PMC article.
-
Role of Glucocorticoids in Metabolic Dysfunction-Associated Steatotic Liver Disease.Curr Obes Rep. 2024 Jun;13(2):242-255. doi: 10.1007/s13679-024-00556-1. Epub 2024 Mar 8. Curr Obes Rep. 2024. PMID: 38459229 Free PMC article. Review.
-
Risk Factors for MAFLD and Advanced Liver Fibrosis in Adult-Onset Craniopharyngioma Patients: A Cross-Sectional Study.Diabetes Metab Syndr Obes. 2025 Mar 25;18:859-871. doi: 10.2147/DMSO.S504968. eCollection 2025. Diabetes Metab Syndr Obes. 2025. PMID: 40161287 Free PMC article.
-
Long-term use of continuous subcutaneous hydrocortisone infusion therapy in patients with congenital adrenal hyperplasia.Clin Endocrinol (Oxf). 2018 Oct;89(4):399-407. doi: 10.1111/cen.13813. Epub 2018 Aug 8. Clin Endocrinol (Oxf). 2018. PMID: 30003563 Free PMC article.
References
-
- de Alwis NM, Day CP. Non-alcoholic fatty liver disease: the mist gradually clears. J Hepatol. 2008;48(Suppl 1):S104–S112. - PubMed
-
- Rockall AG, Sohaib SA, Evans D, Kaltsas G, Isidori AM, et al. Hepatic steatosis in Cushing's syndrome: a radiological assessment using computed tomography. Eur J Endocrinol. 2003;149:543–548. - PubMed
-
- Dourakis SP, Sevastianos VA, Kaliopi P. Acute severe steatohepatitis related to prednisolone therapy. Am J Gastroenterol. 2002;97:1074–1075. - PubMed
-
- Nanki T, Koike R, Miyasaka N. Subacute severe steatohepatitis during prednisolone therapy for systemic lupus erythematosis. Am J Gastroenterol. 1999;94:3379. - PubMed
-
- Hellerstein MK. De novo lipogenesis in humans: metabolic and regulatory aspects. Eur J Clin Nutr. 1999;53(Suppl 1):S53–S65. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

