CCN2/connective tissue growth factor is essential for pericyte adhesion and endothelial basement membrane formation during angiogenesis
- PMID: 22363445
- PMCID: PMC3282727
- DOI: 10.1371/journal.pone.0030562
CCN2/connective tissue growth factor is essential for pericyte adhesion and endothelial basement membrane formation during angiogenesis
Abstract
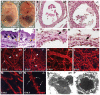
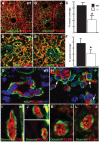
CCN2/Connective Tissue Growth Factor (CTGF) is a matricellular protein that regulates cell adhesion, migration, and survival. CCN2 is best known for its ability to promote fibrosis by mediating the ability of transforming growth factor β (TGFβ) to induce excess extracellular matrix production. In addition to its role in pathological processes, CCN2 is required for chondrogenesis. CCN2 is also highly expressed during development in endothelial cells, suggesting a role in angiogenesis. The potential role of CCN2 in angiogenesis is unclear, however, as both pro- and anti-angiogenic effects have been reported. Here, through analysis of Ccn2-deficient mice, we show that CCN2 is required for stable association and retention of pericytes by endothelial cells. PDGF signaling and the establishment of the endothelial basement membrane are required for pericytes recruitment and retention. CCN2 induced PDGF-B expression in endothelial cells, and potentiated PDGF-B-mediated Akt signaling in mural (vascular smooth muscle/pericyte) cells. In addition, CCN2 induced the production of endothelial basement membrane components in vitro, and was required for their expression in vivo. Overall, these results highlight CCN2 as an essential mediator of vascular remodeling by regulating endothelial-pericyte interactions. Although most studies of CCN2 function have focused on effects of CCN2 overexpression on the interstitial extracellular matrix, the results presented here show that CCN2 is required for the normal production of vascular basement membranes.
Conflict of interest statement
Figures



References
-
- Brigstock DR. The CCN family: a new stimulus package. J Endocrinol. 2003;178:169–175. - PubMed
-
- Bork P. The modular architecture of a new family of growth regulators related to connective tissue growth factor. FEBS Lett. 1993;327:125–130. - PubMed
-
- Vorwerk P, Hohmann B, Oh Y, Rosenfeld RG, Shymko RM. Binding properties of insulin-like growth factor binding protein-3 (IGFBP-3), IGFBP-3 N- and C-terminal fragments, and structurally related proteins mac25 and connective tissue growth factor measured using a biosensor. Endocrinology. 2002;143:1677–1685. - PubMed
-
- Hashimoto G, Inoki I, Fujii Y, Aoki T, Ikeda E, et al. Matrix metalloproteinases cleave connective tissue growth factor and reactivate angiogenic activity of vascular endothelial growth factor 165. J Biol Chem. 2002;277:36288–36295. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

