Origins of the tumor microenvironment: quantitative assessment of adipose-derived and bone marrow-derived stroma
- PMID: 22363446
- PMCID: PMC3282707
- DOI: 10.1371/journal.pone.0030563
Origins of the tumor microenvironment: quantitative assessment of adipose-derived and bone marrow-derived stroma
Abstract
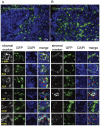
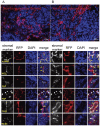
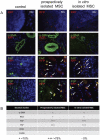
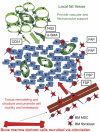
To meet the requirements for rapid tumor growth, a complex array of non-neoplastic cells are recruited to the tumor microenvironment. These cells facilitate tumor development by providing matrices, cytokines, growth factors, as well as vascular networks for nutrient and waste exchange, however their precise origins remain unclear. Through multicolored tissue transplant procedures; we have quantitatively determined the contribution of bone marrow-derived and adipose-derived cells to stromal populations within syngeneic ovarian and breast murine tumors. Our results indicate that subpopulations of tumor-associated fibroblasts (TAFs) are recruited from two distinct sources. The majority of fibroblast specific protein (FSP) positive and fibroblast activation protein (FAP) positive TAFs originate from mesenchymal stem/stromal cells (MSC) located in bone marrow sources, whereas most vascular and fibrovascular stroma (pericytes, α-SMA(+) myofibroblasts, and endothelial cells) originates from neighboring adipose tissue. These results highlight the capacity for tumors to utilize multiple sources of structural cells in a systematic and discriminative manner.
Conflict of interest statement
Figures


References
-
- Udagawa T, Puder M, Wood M, Schaefer BC, D'Amato RJ. Analysis of tumor-associated stromal cells using SCID GFP transgenic mice: contribution of local and bone marrow-derived host cells. FASEB J. 2006;20:95–102. - PubMed
-
- Studeny M, Marini FC, Zompetta C, Champlin RE, Filder IJ, et al. Bone marrow derived mesenchymal stem cells serve as precursors for stromal fibroblasts in malignant tumors and show potential for cancer therapy. Blood. 2001;9:697a.
Publication types
MeSH terms
Substances
Grants and funding
- P30 CA016672/CA/NCI NIH HHS/United States
- CA49639/CA/NCI NIH HHS/United States
- CA16672/CA/NCI NIH HHS/United States
- CA109451/CA/NCI NIH HHS/United States
- R01 CA109451/CA/NCI NIH HHS/United States
- CA55164/CA/NCI NIH HHS/United States
- P50 CA083639/CA/NCI NIH HHS/United States
- R01CA109451/CA/NCI NIH HHS/United States
- CA-116199/CA/NCI NIH HHS/United States
- P01 CA055164/CA/NCI NIH HHS/United States
- RC1 CA146381/CA/NCI NIH HHS/United States
- RC1CA146381/CA/NCI NIH HHS/United States
- P01 CA049639/CA/NCI NIH HHS/United States
- R01 NS069964/NS/NINDS NIH HHS/United States
- P50CA083639/CA/NCI NIH HHS/United States
- R01NS06994/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

