Anandamide induces sperm release from oviductal epithelia through nitric oxide pathway in bovines
- PMID: 22363468
- PMCID: PMC3281848
- DOI: 10.1371/journal.pone.0030671
Anandamide induces sperm release from oviductal epithelia through nitric oxide pathway in bovines
Abstract
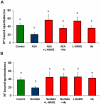
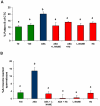
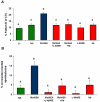
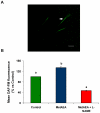
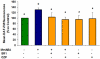
Mammalian spermatozoa are not able to fertilize an egg immediately upon ejaculation. They acquire this ability during their transit through the female genital tract in a process known as capacitation. The mammalian oviduct acts as a functional sperm reservoir providing a suitable environment that allows the maintenance of sperm fertilization competence until ovulation occurs. After ovulation, spermatozoa are gradually released from the oviductal reservoir in the caudal isthmus and ascend to the site of fertilization. Capacitating-related changes in sperm plasma membrane seem to be responsible for sperm release from oviductal epithelium. Anandamide is a lipid mediator that participates in the regulation of several female and male reproductive functions. Previously we have demonstrated that anandamide was capable to release spermatozoa from oviductal epithelia by induction of sperm capacitation in bovines. In the present work we studied whether anandamide might exert its effect by activating the nitric oxide (NO) pathway since this molecule has been described as a capacitating agent in spermatozoa from different species. First, we demonstrated that 1 µM NOC-18, a NO donor, and 10 mM L-Arginine, NO synthase substrate, induced the release of spermatozoa from the oviductal epithelia. Then, we observed that the anandamide effect on sperm oviduct interaction was reversed by the addition of 1 µM L-NAME, a NO synthase inhibitor, or 30 µg/ml Hemoglobin, a NO scavenger. We also demonstrated that the induction of bull sperm capacitation by nanomolar concentrations of R(+)-methanandamide or anandamide was inhibited by adding L-NAME or Hemoglobin. To study whether anandamide is able to produce NO, we measured this compound in both sperm and oviductal cells. We observed that anandamide increased the levels of NO in spermatozoa, but not in oviductal cells. These findings suggest that anandamide regulates the sperm release from oviductal epithelia probably by activating the NO pathway during sperm capacitation.
Conflict of interest statement
Figures
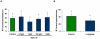

Similar articles
-
Anandamide capacitates bull spermatozoa through CB1 and TRPV1 activation.PLoS One. 2011 Feb 11;6(2):e16993. doi: 10.1371/journal.pone.0016993. PLoS One. 2011. PMID: 21347292 Free PMC article.
-
Sperm Release From the Oviductal Epithelium Depends on Ca(2+) Influx Upon Activation of CB1 and TRPV1 by Anandamide.J Cell Biochem. 2016 Feb;117(2):320-33. doi: 10.1002/jcb.25273. J Cell Biochem. 2016. PMID: 26129689
-
The endocannabinoid system in bull sperm and bovine oviductal epithelium: role of anandamide in sperm-oviduct interaction.Reproduction. 2009 Mar;137(3):403-14. doi: 10.1530/REP-08-0204. Epub 2008 Nov 28. Reproduction. 2009. PMID: 19042982
-
A tale of two cells: endocannabinoid-signaling regulates functions of neurons and sperm.Biol Reprod. 2005 Dec;73(6):1078-86. doi: 10.1095/biolreprod.105.043273. Epub 2005 Aug 24. Biol Reprod. 2005. PMID: 16120829 Review.
-
Molecules involved in sperm-oviduct adhesion and release.Theriogenology. 2010 Apr 1;73(6):796-801. doi: 10.1016/j.theriogenology.2009.07.005. Epub 2009 Aug 13. Theriogenology. 2010. PMID: 19682733 Review.
Cited by
-
Storage and release of spermatozoa from the pre-uterine tube reservoir.PLoS One. 2013;8(2):e57006. doi: 10.1371/journal.pone.0057006. Epub 2013 Feb 25. PLoS One. 2013. PMID: 23451135 Free PMC article.
-
Crosstalk Between Nitric Oxide and Endocannabinoid Signaling Pathways in Normal and Pathological Placentation.Front Physiol. 2018 Dec 4;9:1699. doi: 10.3389/fphys.2018.01699. eCollection 2018. Front Physiol. 2018. PMID: 30564135 Free PMC article. Review.
-
Sub-fertility in crossbred bulls: deciphering testicular level transcriptomic alterations between zebu (Bos indicus) and crossbred (Bos taurus x Bos indicus) bulls.BMC Genomics. 2020 Jul 21;21(1):502. doi: 10.1186/s12864-020-06907-1. BMC Genomics. 2020. PMID: 32693775 Free PMC article.
-
Review: The epic journey of sperm through the female reproductive tract.Animal. 2018 Jun;12(s1):s110-s120. doi: 10.1017/S1751731118000526. Epub 2018 Mar 19. Animal. 2018. PMID: 29551099 Free PMC article. Review.
-
Cyclic AMP efflux through MRP4 regulates actin dynamics signalling pathway and sperm motility in bovines.Sci Rep. 2020 Sep 24;10(1):15619. doi: 10.1038/s41598-020-72425-5. Sci Rep. 2020. PMID: 32973195 Free PMC article.
References
-
- Suarez SS. Regulation of sperm storage and movement in the mammalian oviduct. Int J Dev Biol. 2008;52(5–6):455–462. - PubMed
-
- Hunter RHF. Sperm release from oviduct epithelial binding is controlled hormonally by peri-ovularoty Graafian follicles. Mol Reprod Develop. 2008;75:167–174. - PubMed
-
- Visconti PE, Westbrook VA, Chertihin O, Demarco I, Sleight S, et al. Novel signaling pathways involved in sperm acquisition of fertilizing capacity. J Reprod Immunol. 2002;53(1–2):133–150. - PubMed
-
- Herrero MB, de Lamirande E, Gagnon C. Nitric oxide is a signaling molecule in spermatozoa. Curr Pharm Des. 2003;9(5):419–425. - PubMed
-
- Talevi R, Gualtieri R. Sulfated glycoconjugates are powerful modulators of bovine sperm adhesion and release from the oviductal epithelium in vitro. Biol Reprod. 2001;64(2):491–498. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

