TIM-3 expression characterizes regulatory T cells in tumor tissues and is associated with lung cancer progression
- PMID: 22363469
- PMCID: PMC3281852
- DOI: 10.1371/journal.pone.0030676
TIM-3 expression characterizes regulatory T cells in tumor tissues and is associated with lung cancer progression
Abstract
Background: T cell immunoglobulin-3 (TIM-3) has been established as a negative regulatory molecule and plays a critical role in immune tolerance. TIM-3 is upregulated in exhausted CD8(+) T cells in both chronic infection and tumor. However, the nature of TIM-3(+)CD4(+) T cells in the tumor microenvironment is unclear. This study is to characterize TIM-3 expressing lymphocytes within human lung cancer tissues and establish clinical significance of TIM-3 expression in lung cancer progression.
Methodology: A total of 51 human lung cancer tissue specimens were obtained from pathologically confirmed and newly diagnosed non-small cell lung cancer (NSCLC) patients. Leukocytes from tumor tissues, distal normal lung tissues, and peripheral blood mononuclear cells (PBMC) were analyzed for TIM-3 surface expression by flow cytometry. TIM-3 expression on tumor-infiltrating lymphocytes (TILs) was correlated with clinicopathological parameters.
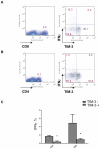
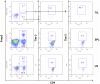
Conclusions: TIM-3 is highly upregulated on both CD4(+) and CD8(+) TILs from human lung cancer tissues but negligibly expressed on T cells from patients' peripheral blood. Frequencies of IFN-γ(+) cells were reduced in TIM-3(+)CD8(+) TILs compared to TIM-3(-)CD8(+) TILs. However, the level of TIM-3 expression on CD8(+) TILs failed to associate with any clinical pathological parameter. Interestingly, we found that approximately 70% of TIM-3(+)CD4(+) TILs expressed FOXP3 and about 60% of FOXP3(+) TILs were TIM-3(+). Importantly, TIM-3 expression on CD4(+) T cells correlated with poor clinicopathological parameters of NSCLC such as nodal metastasis and advanced cancer stages. Our study reveals a new role of TIM-3 as an important immune regulator in the tumor microenvironment via its predominant expression in regulatory T cells.
Conflict of interest statement
Figures



References
-
- Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–1964. - PubMed
-
- Zou W. Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nat Rev Cancer. 2005;5:263–274. - PubMed
-
- Zou W. Regulatory T cells, tumour immunity and immunotherapy. Nat Rev Immunol. 2006;6:295–307. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

