A heterogeneous in vitro three dimensional model of tumour-stroma interactions regulating sprouting angiogenesis
- PMID: 22363483
- PMCID: PMC3282728
- DOI: 10.1371/journal.pone.0030753
A heterogeneous in vitro three dimensional model of tumour-stroma interactions regulating sprouting angiogenesis
Abstract
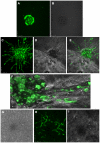
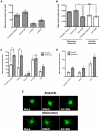
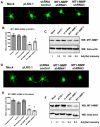
Angiogenesis, the formation of new blood vessels, is an essential process for tumour progression and is an area of significant therapeutic interest. Different in vitro systems and more complex in vivo systems have been described for the study of tumour angiogenesis. However, there are few human 3D in vitro systems described to date which mimic the cellular heterogeneity and complexity of angiogenesis within the tumour microenvironment. In this study we describe the Minitumour model--a 3 dimensional human spheroid-based system consisting of endothelial cells and fibroblasts in co-culture with the breast cancer cell line MDA-MB-231, for the study of tumour angiogenesis in vitro. After implantation in collagen-I gels, Minitumour spheroids form quantifiable endothelial capillary-like structures. The endothelial cell pre-capillary sprouts are supported by the fibroblasts, which act as mural cells, and their growth is increased by the presence of cancer cells. Characterisation of the Minitumour model using small molecule inhibitors and inhibitory antibodies show that endothelial sprout formation is dependent on growth factors and cytokines known to be important for tumour angiogenesis. The model also shows a response to anti-angiogenic agents similar to previously described in vivo data. We demonstrate that independent manipulation of the different cell types is possible, using common molecular techniques, before incorporation into the model. This aspect of Minitumour spheroid analysis makes this model ideal for high content studies of gene function in individual cell types, allowing for the dissection of their roles in cell-cell interactions. Finally, using this technique, we were able to show the requirement of the metalloproteinase MT1-MMP in endothelial cells and fibroblasts, but not cancer cells, for sprouting angiogenesis.
Conflict of interest statement
Figures


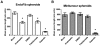

Similar articles
-
Inhibition of vascular endothelial growth factor-associated tyrosine kinase activity with SU5416 blocks sprouting in the microvascular endothelial cell spheroid model of angiogenesis.Microvasc Res. 2002 May;63(3):304-15. doi: 10.1006/mvre.2001.2383. Microvasc Res. 2002. PMID: 11969307
-
RAIN-Droplet: a novel 3D in vitro angiogenesis model.Lab Invest. 2012 Jul;92(7):988-98. doi: 10.1038/labinvest.2012.77. Epub 2012 May 7. Lab Invest. 2012. PMID: 22565576 Free PMC article.
-
Three-dimensional Angiogenesis Assay System using Co-culture Spheroids Formed by Endothelial Colony Forming Cells and Mesenchymal Stem Cells.J Vis Exp. 2019 Sep 18;(151). doi: 10.3791/60032. J Vis Exp. 2019. PMID: 31609345
-
Vascular endothelial cell growth factor (VEGF), an emerging target for cancer chemotherapy.Curr Med Chem Anticancer Agents. 2003 Mar;3(2):95-117. doi: 10.2174/1568011033353452. Curr Med Chem Anticancer Agents. 2003. PMID: 12678905 Review.
-
Tumours can adapt to anti-angiogenic therapy depending on the stromal context: lessons from endothelial cell biology.Eur J Cell Biol. 2006 Feb;85(2):61-8. doi: 10.1016/j.ejcb.2005.10.003. Epub 2005 Nov 11. Eur J Cell Biol. 2006. PMID: 16439306 Review.
Cited by
-
Heralding a new paradigm in 3D tumor modeling.Biomaterials. 2016 Nov;108:197-213. doi: 10.1016/j.biomaterials.2016.08.052. Epub 2016 Sep 2. Biomaterials. 2016. PMID: 27639438 Free PMC article. Review.
-
Angiogenic biomaterials to promote therapeutic regeneration and investigate disease progression.Biomaterials. 2020 Oct;255:120207. doi: 10.1016/j.biomaterials.2020.120207. Epub 2020 Jun 14. Biomaterials. 2020. PMID: 32569868 Free PMC article. Review.
-
A 3D Human Renal Cell Carcinoma-on-a-Chip for the Study of Tumor Angiogenesis.Neoplasia. 2018 Jun;20(6):610-620. doi: 10.1016/j.neo.2018.02.011. Epub 2018 May 7. Neoplasia. 2018. PMID: 29747161 Free PMC article.
-
Liquid-based three-dimensional tumor models for cancer research and drug discovery.Exp Biol Med (Maywood). 2016 May;241(9):939-54. doi: 10.1177/1535370216643772. Epub 2016 Apr 11. Exp Biol Med (Maywood). 2016. PMID: 27072562 Free PMC article. Review.
-
Modeling tumor cell adaptations to hypoxia in multicellular tumor spheroids.J Exp Clin Cancer Res. 2017 Aug 3;36(1):102. doi: 10.1186/s13046-017-0570-9. J Exp Clin Cancer Res. 2017. PMID: 28774341 Free PMC article. Review.
References
-
- Tlsty TD, Coussens LM. Tumor stroma and regulation of cancer development. Annu Rev Pathol. 2006;1:119–150. - PubMed
-
- Nyberg P, Salo T, Kalluri R. Tumor microenvironment and angiogenesis. Front Biosci. 2008;13:6537–6553. - PubMed
-
- Albini A, Sporn MB. The tumour microenvironment as a target for chemoprevention. Nat Rev Cancer. 2007;7:139–147. - PubMed
-
- Mueller MM, Fusenig NE. Friends or foes - bipolar effects of the tumour stroma in cancer. Nat Rev Cancer. 2004;4:839–849. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

