Bone marrow osteoblast damage by chemotherapeutic agents
- PMID: 22363485
- PMCID: PMC3281873
- DOI: 10.1371/journal.pone.0030758
Bone marrow osteoblast damage by chemotherapeutic agents
Abstract
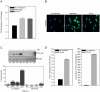
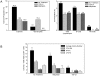
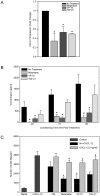
Hematopoietic reconstitution, following bone marrow or stem cell transplantation, requires a microenvironment niche capable of supporting both immature progenitors and stem cells with the capacity to differentiate and expand. Osteoblasts comprise one important component of this niche. We determined that treatment of human primary osteoblasts (HOB) with melphalan or VP-16 resulted in increased phospho-Smad2, consistent with increased TGF-β1 activity. This increase was coincident with reduced HOB capacity to support immature B lineage cell chemotaxis and adherence. The supportive deficit was not limited to committed progenitor cells, as human embryonic stem cells (hESC) or human CD34+ bone marrow cells co-cultured with HOB pre-exposed to melphalan, VP-16 or rTGF-β1 had profiles distinct from the same populations co-cultured with untreated HOB. Functional support deficits were downstream of changes in HOB gene expression profiles following chemotherapy exposure. Melphalan and VP-16 induced damage of HOB suggests vulnerability of this critical niche to therapeutic agents frequently utilized in pre-transplant regimens and suggests that dose escalated chemotherapy may contribute to post-transplantation hematopoietic deficits by damaging structural components of this supportive niche.
Conflict of interest statement
Figures
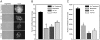
Similar articles
-
Bone marrow osteoblast vulnerability to chemotherapy.Eur J Haematol. 2013 Jun;90(6):469-78. doi: 10.1111/ejh.12109. Epub 2013 May 3. Eur J Haematol. 2013. PMID: 23551534 Free PMC article.
-
Melphalan exposure induces an interleukin-6 deficit in bone marrow stromal cells and osteoblasts.Cytokine. 2012 May;58(2):245-52. doi: 10.1016/j.cyto.2012.01.012. Epub 2012 Feb 21. Cytokine. 2012. PMID: 22356805 Free PMC article.
-
Reduced bone marrow stem cell pool and progenitor mobilisation in multiple myeloma after melphalan treatment.Med Oncol. 1999 Dec;16(4):245-54. doi: 10.1007/BF02785870. Med Oncol. 1999. PMID: 10618687
-
Regulation of hematopoietic stem cells by bone marrow stromal cells.Trends Immunol. 2014 Jan;35(1):32-7. doi: 10.1016/j.it.2013.10.002. Epub 2013 Nov 5. Trends Immunol. 2014. PMID: 24210164 Free PMC article. Review.
-
The role of granulocyte colony-stimulating factor in mobilization and transplantation of peripheral blood progenitor and stem cells.Cytokines Mol Ther. 1995 Dec;1(4):249-70. Cytokines Mol Ther. 1995. PMID: 9384679 Review.
Cited by
-
Bone marrow osteoblast vulnerability to chemotherapy.Eur J Haematol. 2013 Jun;90(6):469-78. doi: 10.1111/ejh.12109. Epub 2013 May 3. Eur J Haematol. 2013. PMID: 23551534 Free PMC article.
-
Clonality in context: hematopoietic clones in their marrow environment.Blood. 2017 Nov 30;130(22):2363-2372. doi: 10.1182/blood-2017-07-794362. Epub 2017 Oct 18. Blood. 2017. PMID: 29046282 Free PMC article.
-
The skeletal impact of the chemotherapeutic agent etoposide.Osteoporos Int. 2017 Aug;28(8):2321-2333. doi: 10.1007/s00198-017-4032-1. Epub 2017 Apr 20. Osteoporos Int. 2017. PMID: 28429052 Free PMC article.
-
PTHrP attenuates osteoblast cell death and apoptosis induced by a novel class of anti-cancer agents.Endocrine. 2016 Mar;51(3):534-44. doi: 10.1007/s12020-015-0699-2. Epub 2015 Aug 11. Endocrine. 2016. PMID: 26260694
-
Chemotherapy-induced Dkk-1 expression by primary human mesenchymal stem cells is p53 dependent.Med Oncol. 2016 Oct;33(10):113. doi: 10.1007/s12032-016-0826-9. Epub 2016 Sep 1. Med Oncol. 2016. PMID: 27586146 Free PMC article.
References
-
- Schofield R. The relationship between the spleen colony-forming cell and the haemopoietic stem cell. Blood Cells. 1978;4:7–25. - PubMed
-
- Taichman RS, Emerson SG. The role of osteoblasts in the hematopoietic microenvironment. Stem Cells. 1998;16:7–15. - PubMed
-
- Nilsson SK, Johnston HM, Coverdale JA. Spatial localization of transplanted hemopoietic stem cells: inferences for the localization of stem cell niches. Blood. 2001;97:2293–2299. - PubMed
-
- Xie Y, Yin T, Wiegraebe W, He XC, Miller D, et al. Detection of functional haematopoietic stem cell niche using real-time imaging. Nature. 2009;457:97–101. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

