Differences in MEF2 and NFAT transcriptional pathways according to human heart failure aetiology
- PMID: 22363514
- PMCID: PMC3281902
- DOI: 10.1371/journal.pone.0030915
Differences in MEF2 and NFAT transcriptional pathways according to human heart failure aetiology
Abstract
Background: Ca(2+) handling machinery modulates the activation of cardiac transcription pathways involved in heart failure (HF). The present study investigated the effect of HF aetiology on Ca(+2) handling proteins and NFAT1, MEF2C and GATA4 (transcription factors) in the same cardiac tissue.
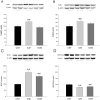
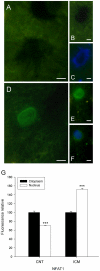
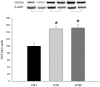
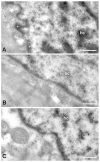
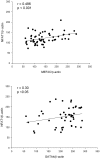
Methodology and principal findings: A total of 83 hearts from ischemic (ICM, n = 43) and dilated (DCM, n = 31) patients undergoing heart transplantation and controls (CNT, n = 9) were analyzed by western blotting. Subcellular distribution was analyzed by fluorescence and electron microscopy. When we compared Ca(+2) handling proteins according to HF aetiology, ICM showed higher levels of calmodulin (24%, p<0.01), calcineurin (26%, p<0.01) and Ca(2+)/Calmodulin-dependent kinase II (CaMKIIδ(b) nuclear isoform 62%, p<0.001) than the CNT group. However, these proteins in DCM did not significantly increase. Furthermore, ICM showed a significant elevation in MEF2C (33%, p<0.01), and GATA4 (49%, p<0.05); also NFAT1 (66%, p<0.001) was increased, producing the resultant translocation of this transcriptional factor into the nuclei. These results were supported by fluorescence and electron microscopy analysis. Whereas, DCM only had a significant increase in GATA4 (52%, p<0.05). Correlations between NFAT1 and MEF2C in both groups (ICM r = 0.38 and DCM r = 0.59, p<0.05 and p<0.01, respectively) were found; only ICM showed a correlation between GATA4 and NFAT1 (r = 0.37, p<0.05).
Conclusions/significance: This study shows an increase of Ca(2+) handling machinery synthesis and their cardiac transcription pathways in HF, being more markedly increased in ICM. Furthermore, there is a significant association between MEF2, NFAT1 and GATA4. These proteins could be therapeutic targets to improve myocardial function.
Conflict of interest statement
Figures
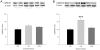
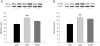
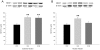
Similar articles
-
Calcium-regulated transcriptional pathways in the normal and pathologic heart.IUBMB Life. 2011 Oct;63(10):847-55. doi: 10.1002/iub.545. Epub 2011 Sep 7. IUBMB Life. 2011. PMID: 21901815 Review.
-
Dilated cardiomyopathy mutations in thin-filament regulatory proteins reduce contractility, suppress systolic Ca2+, and activate NFAT and Akt signaling.Am J Physiol Heart Circ Physiol. 2020 Aug 1;319(2):H306-H319. doi: 10.1152/ajpheart.00272.2020. Epub 2020 Jul 3. Am J Physiol Heart Circ Physiol. 2020. PMID: 32618513 Free PMC article.
-
Downregulation of myocardial myocyte enhancer factor 2C and myocyte enhancer factor 2C-regulated gene expression in diabetic patients with nonischemic heart failure.Circulation. 2002 Jul 23;106(4):407-11. doi: 10.1161/01.cir.0000026392.80723.dc. Circulation. 2002. PMID: 12135937
-
Activation of Na+/H+ exchanger 1 is sufficient to generate Ca2+ signals that induce cardiac hypertrophy and heart failure.Circ Res. 2008 Oct 10;103(8):891-9. doi: 10.1161/CIRCRESAHA.108.175141. Epub 2008 Sep 5. Circ Res. 2008. PMID: 18776042
-
Role of oxidants on calcium and sodium movement in healthy and diseased cardiac myocytes.Free Radic Biol Med. 2013 Oct;63:338-49. doi: 10.1016/j.freeradbiomed.2013.05.035. Epub 2013 Jun 1. Free Radic Biol Med. 2013. PMID: 23732518 Review.
Cited by
-
Increased expression of fatty-acid and calcium metabolism genes in failing human heart.PLoS One. 2012;7(6):e37505. doi: 10.1371/journal.pone.0037505. Epub 2012 Jun 6. PLoS One. 2012. PMID: 22701570 Free PMC article.
-
MEF2C repressor variant deregulation leads to cell cycle re-entry and development of heart failure.EBioMedicine. 2020 Jan;51:102571. doi: 10.1016/j.ebiom.2019.11.032. Epub 2020 Jan 3. EBioMedicine. 2020. PMID: 31911274 Free PMC article.
-
Androgen-Regulated Cardiac Metabolism in Aging Men.Front Endocrinol (Lausanne). 2020 May 15;11:316. doi: 10.3389/fendo.2020.00316. eCollection 2020. Front Endocrinol (Lausanne). 2020. PMID: 32499759 Free PMC article. Review.
-
MEF-2 isoforms' (A-D) roles in development and tumorigenesis.Oncotarget. 2019 Apr 12;10(28):2755-2787. doi: 10.18632/oncotarget.26763. eCollection 2019 Apr 12. Oncotarget. 2019. PMID: 31105874 Free PMC article. Review.
-
Visualizing CaMKII and CaM activity: a paradigm of compartmentalized signaling.J Mol Med (Berl). 2013 Aug;91(8):907-16. doi: 10.1007/s00109-013-1060-y. Epub 2013 Jun 18. J Mol Med (Berl). 2013. PMID: 23775230 Free PMC article. Review.
References
-
- Cohn JN, Ferrari R, Sharpe N. Cardiac remodeling–concepts and clinical implications: a consensus paper from an international forum on cardiac remodeling. Behalf of an International Forum on Cardiac Remodeling. J Am Coll Cardiol. 2000;35:569–582. - PubMed
-
- Hill JA, Olson EN. Cardiac plasticity. N Engl J Med. 2008;358:1370–80. - PubMed
-
- Sadoshima J, Izumo S. The cellular and molecular response of cardiac myocytes to mechanical stress. Annu Rev Physiol. 1997;59:551–571. - PubMed
-
- Beuckelmann DJ, Näbauer M, Erdmann E. Intracellular calcium handling in isolated ventricular myocytes from patients with terminal heart failure. Circulation. 1992;85:1046–1055. - PubMed
-
- Hasenfuss G, Pieske B. Calcium cycling in congestive heart failure. J Mol Cell Cardiol. 2002;34:951–969. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

