An N-ethyl-N-nitrosourea (ENU)-induced dominant negative mutation in the JAK3 kinase protects against cerebral malaria
- PMID: 22363534
- PMCID: PMC3283600
- DOI: 10.1371/journal.pone.0031012
An N-ethyl-N-nitrosourea (ENU)-induced dominant negative mutation in the JAK3 kinase protects against cerebral malaria
Abstract
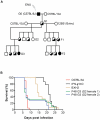
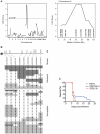
Cerebral malaria (CM) is a lethal neurological complication of malaria. We implemented a genome-wide screen in mutagenized mice to identify host proteins involved in CM pathogenesis and whose inhibition may be of therapeutic value. One pedigree (P48) segregated a resistance trait whose CM-protective effect was fully penetrant, mapped to chromosome 8, and identified by genome sequencing as homozygosity for a mis-sense mutation (W81R) in the FERM domain of Janus-associated kinase 3 (Jak3). The causative effect of Jak3(W81R) was verified by complementation testing in Jak3(W81R/-) double heterozygotes that were fully protected against CM. Jak3(W81R) homozygotes showed defects in thymic development with depletion of CD8(+) T cell, B cell, and NK cell compartments, and defective T cell-dependent production of IFN-γ. Adoptive transfer of normal splenocytes abrogates CM resistance in Jak3(W81R) homozygotes, an effect attributed to the CD8(+) T cells. Jak3(W81R) behaves as a dominant negative variant, with significant CM resistance of Jak3(W81R/+) heterozygotes, compared to CM-susceptible Jak3(+/+) and Jak3(+/-) controls. CM resistance in Jak3(W81R/+) heterozygotes occurs in presence of normal T, B and NK cell numbers. These findings highlight the pathological role of CD8(+) T cells and Jak3-dependent IFN-γ-mediated Th1 responses in CM pathogenesis.
Conflict of interest statement
Figures





References
-
- WHO website. Available: http://www.who.int/features/factfiles/malaria/en/index.html. Accessed 2009 March.
-
- Hunt NH, Grau GE. Cytokines: accelerators and brakes in the pathogenesis of cerebral malaria. Trends Immunol. 2003;24:491–499. - PubMed
-
- Bongfen SE, Laroque A, Berghout J, Gros P. Genetic and genomic analyses of host-pathogen interactions in malaria. Trends Parasitol. 2009;25:417–422. - PubMed
-
- Kwiatkowski D. Genetic susceptibility to malaria getting complex. Curr Opin Genet Dev. 2000;10:320–324. - PubMed
-
- Weatherall DJ. Genetic variation and susceptibility to infection: the red cell and malaria. Br J Haematol. 2008;141:276–286. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

