Activation of the AMP-activated protein kinase (AMPK) by nitrated lipids in endothelial cells
- PMID: 22363546
- PMCID: PMC3281919
- DOI: 10.1371/journal.pone.0031056
Activation of the AMP-activated protein kinase (AMPK) by nitrated lipids in endothelial cells
Retraction in
-
Retraction: Activation of the AMP-Activated Protein Kinase (AMPK) by Nitrated Lipids in Endothelial Cells.PLoS One. 2020 May 19;15(5):e0233408. doi: 10.1371/journal.pone.0233408. eCollection 2020. PLoS One. 2020. PMID: 32428040 Free PMC article. No abstract available.
Abstract
The AMP-activated protein kinase (AMPK) is an important regulator of endothelial metabolic and functional homeostasis. Here, we examined the regulation of AMPK by nitrated oleic acid (OA-NO(2)) and investigated the implications in endothelial function. Treatment of bovine aortic endothelial cells (BAECs) with OA-NO(2) induced a significant increase in both AMPK-Thr172 phosphorylation and AMPK activity as well as upregulation of heme oxygenase (HO)-1 and hypoxia-inducible factor (HIF)-1α. Pharmacologic inhibition or genetic ablation of HO-1 or HIF-1α abolished OA-NO(2)-induced AMPK phosphorylation. OA-NO(2) induced a dramatic increase in extracellular signal-regulated kinase (ERK)1/2 phosphorylation that was abrogated by the HO-1 inhibitor, zinc deuteroporphyrin IX 2,4-bis-ethylene glycol (ZnBG). Inhibition of ERK1/2 using UO126 or PD98059 reduced but did not abolish OA-NO(2)-induced HIF-1α upregulation, suggesting that OA-NO(2)/HO-1-initiated HIF-1α induction is partially dependent on ERK1/2 activity. In addition, OA-NO(2) enhanced endothelial intracellular Ca(2+), an effect that was inhibited by the HIF-1α inhibitor, YC-1, and by HIF-1α siRNA. These results implicate the involvement of HIF-1α. Experiments using the Ca(2+)/calmodulin-dependent protein kinase kinase (CaMKK) inhibitor STO-609, the selective CaMKII inhibitor KN-93, and an isoform-specific siRNA demonstrated that OA-NO(2)-induced AMPK phosphorylation was dependent on CaMKKβ. Together, these results demonstrate that OA-NO(2) activates AMPK in endothelial cells via an HO-1-dependent mechanism that increases HIF-1α protein expression and Ca(2+)/CaMKKβ activation.
Conflict of interest statement
Figures
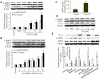
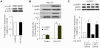
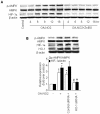

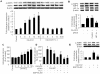

Similar articles
-
Thrombin activates AMP-activated protein kinase in endothelial cells via a pathway involving Ca2+/calmodulin-dependent protein kinase kinase beta.Mol Cell Biol. 2006 Aug;26(16):5933-45. doi: 10.1128/MCB.00383-06. Mol Cell Biol. 2006. PMID: 16880506 Free PMC article.
-
Ramipril protects the endothelium from high glucose-induced dysfunction through CaMKKβ/AMPK and heme oxygenase-1 activation.J Pharmacol Exp Ther. 2014 Jul;350(1):5-13. doi: 10.1124/jpet.114.212928. Epub 2014 Apr 16. J Pharmacol Exp Ther. 2014. PMID: 24741076
-
Rutaecarpine Increases Nitric Oxide Synthesis via eNOS Phosphorylation by TRPV1-Dependent CaMKII and CaMKKβ/AMPK Signaling Pathway in Human Endothelial Cells.Int J Mol Sci. 2021 Aug 30;22(17):9407. doi: 10.3390/ijms22179407. Int J Mol Sci. 2021. PMID: 34502308 Free PMC article.
-
AMP-activated protein kinase activation as a strategy for protecting vascular endothelial function.Clin Exp Pharmacol Physiol. 2008 May;35(5-6):535-45. doi: 10.1111/j.1440-1681.2007.04851.x. Epub 2007 Dec 26. Clin Exp Pharmacol Physiol. 2008. PMID: 18177481 Free PMC article. Review.
-
Molecular Mechanisms Underlying Ca2+/Calmodulin-Dependent Protein Kinase Kinase Signal Transduction.Int J Mol Sci. 2022 Sep 20;23(19):11025. doi: 10.3390/ijms231911025. Int J Mol Sci. 2022. PMID: 36232320 Free PMC article. Review.
Cited by
-
Nitro-Oleic Acid Prevents Hypoxia- and Asymmetric Dimethylarginine-Induced Pulmonary Endothelial Dysfunction.Cardiovasc Drugs Ther. 2016 Dec;30(6):579-586. doi: 10.1007/s10557-016-6700-3. Cardiovasc Drugs Ther. 2016. PMID: 27858190 Free PMC article.
-
(S)-1-α-naphthylmethyl-6,7-dihydroxy-1,2,3,4-tetrahydroisoquinoline (CKD712), promotes wound closure by producing VEGF through HO-1 induction in human dermal fibroblasts and mouse skin.Br J Pharmacol. 2013 Mar;168(6):1485-96. doi: 10.1111/bph.12031. Br J Pharmacol. 2013. PMID: 23088309 Free PMC article.
-
Mitochondria and AMP-activated protein kinase-dependent mechanism of efferocytosis.J Biol Chem. 2013 Sep 6;288(36):26013-26026. doi: 10.1074/jbc.M113.489468. Epub 2013 Jul 29. J Biol Chem. 2013. PMID: 23897815 Free PMC article.
-
Retraction: Activation of the AMP-Activated Protein Kinase (AMPK) by Nitrated Lipids in Endothelial Cells.PLoS One. 2020 May 19;15(5):e0233408. doi: 10.1371/journal.pone.0233408. eCollection 2020. PLoS One. 2020. PMID: 32428040 Free PMC article. No abstract available.
-
Interleukin-11 protects against renal ischemia and reperfusion injury.Am J Physiol Renal Physiol. 2012 Oct 15;303(8):F1216-24. doi: 10.1152/ajprenal.00220.2012. Epub 2012 Aug 1. Am J Physiol Renal Physiol. 2012. PMID: 22859402 Free PMC article.
References
-
- Zou MH, Cohen R, Ullrich V. Peroxynitrite and vascular endothelial dysfunction in diabetes mellitus. Endothelium. 2004;11:89–97. - PubMed
-
- Baker PR, Lin Y, Schopfer FJ, Woodcock SR, Groeger AL, et al. Fatty acid transduction of nitric oxide signaling: multiple nitrated unsaturated fatty acid derivatives exist in human blood and urine and serve as endogenous peroxisome proliferator-activated receptor ligands. J Biol Chem. 2005;280:42464–42475. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

