Brainstem respiratory oscillators develop independently of neuronal migration defects in the Wnt/PCP mouse mutant looptail
- PMID: 22363567
- PMCID: PMC3281908
- DOI: 10.1371/journal.pone.0031140
Brainstem respiratory oscillators develop independently of neuronal migration defects in the Wnt/PCP mouse mutant looptail
Abstract
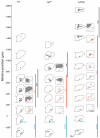
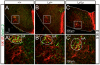
The proper development and maturation of neuronal circuits require precise migration of component neurons from their birthplace (germinal zone) to their final positions. Little is known about the effects of aberrant neuronal position on the functioning of organized neuronal groups, especially in mammals. Here, we investigated the formation and properties of brainstem respiratory neurons in looptail (Lp) mutant mice in which facial motor neurons closely apposed to some respiratory neurons fail to migrate due to loss of function of the Wnt/Planar Cell Polarity (PCP) protein Vangl2. Using calcium imaging and immunostaining on embryonic hindbrain preparations, we found that respiratory neurons constituting the embryonic parafacial oscillator (e-pF) settled at the ventral surface of the medulla in Vangl2(Lp/+) and Vangl2(Lp/Lp) embryos despite the failure of tangential migration of its normally adjacent facial motor nucleus. Anatomically, the e-pF neurons were displaced medially in Lp/+ embryos and rostro-medially Lp/Lp embryos. Pharmacological treatments showed that the e-pF oscillator exhibited characteristic network properties in both Lp/+ and Lp/Lp embryos. Furthermore, using hindbrain slices, we found that the other respiratory oscillator, the preBötzinger complex, was also anatomically and functionally established in Lp mutants. Importantly, the displaced e-pF oscillator established functional connections with the preBötC oscillator in Lp/+ mutants. Our data highlight the robustness of the developmental processes that assemble the neuronal networks mediating an essential physiological function.
Conflict of interest statement
Figures





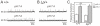

References
-
- Guerrini R, Parrini E. Neuronal migration disorders. Neurobiol Dis. 2010;38:154–166. - PubMed
-
- Verrotti A, Spalice A, Ursitti F, Papetti L, Mariani R, et al. New trends in neuronal migration disorders. Eur J Paediatr Neurol. 2010;14:1–12. - PubMed
-
- Aicardi J. The place of neuronal migration abnormalities in child neurology. Can J Neurol Sci. 1994;21:185–193. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

