Pre-clinical evaluation of a replication-competent recombinant adenovirus serotype 4 vaccine expressing influenza H5 hemagglutinin
- PMID: 22363572
- PMCID: PMC3281928
- DOI: 10.1371/journal.pone.0031177
Pre-clinical evaluation of a replication-competent recombinant adenovirus serotype 4 vaccine expressing influenza H5 hemagglutinin
Abstract
Background: Influenza virus remains a significant health and social concern in part because of newly emerging strains, such as avian H5N1 virus. We have developed a prototype H5N1 vaccine using a recombinant, replication-competent Adenovirus serotype 4 (Ad4) vector, derived from the U.S. military Ad4 vaccine strain, to express the hemagglutinin (HA) gene from A/Vietnam/1194/2004 influenza virus (Ad4-H5-Vtn). Our hypothesis is that a mucosally-delivered replicating Ad4-H5-Vtn recombinant vector will be safe and induce protective immunity against H5N1 influenza virus infection and disease pathogenesis.
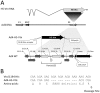
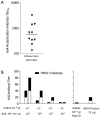
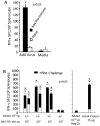
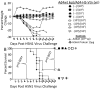
Methodology/principal findings: The Ad4-H5-Vtn vaccine was designed with a partial deletion of the E3 region of Ad4 to accommodate the influenza HA gene. Replication and growth kinetics of the vaccine virus in multiple human cell lines indicated that the vaccine virus is attenuated relative to the wild type virus. Expression of the HA transgene in infected cells was documented by flow cytometry, western blot analysis and induction of HA-specific antibody and cellular immune responses in mice. Of particular note, mice immunized intranasally with the Ad4-H5-Vtn vaccine were protected against lethal H5N1 reassortant viral challenge even in the presence of pre-existing immunity to the Ad4 wild type virus.
Conclusions/significance: Several non-clinical attributes of this vaccine including safety, induction of HA-specific humoral and cellular immunity, and efficacy were demonstrated using an animal model to support Phase 1 clinical trial evaluation of this new vaccine.
Conflict of interest statement
Figures

References
-
- Alexander DJ. An overview of the epidemiology of avian influenza. Vaccine. 2007;25:5637–5644. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

