Recombinant lysyl oxidase propeptide protein inhibits growth and promotes apoptosis of pre-existing murine breast cancer xenografts
- PMID: 22363577
- PMCID: PMC3280126
- DOI: 10.1371/journal.pone.0031188
Recombinant lysyl oxidase propeptide protein inhibits growth and promotes apoptosis of pre-existing murine breast cancer xenografts
Abstract
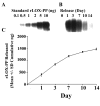
Lysyl oxidase propeptide (LOX-PP) ectopic overexpression inhibits the growth of cancer xenografts. Here the ability and mode of action of purified recombinant LOX-PP (rLOX-PP) protein to inhibit the growth of pre-existing xenografts was determined. Experimental approaches employed were direct intratumoral injection (i.t.) of rLOX-PP protein into murine breast cancer NF639 xenografts, and application of a slow release formulation of rLOX-PP implanted adjacent to tumors in NCR nu/nu mice (n = 10). Tumors were monitored for growth, and after sacrifice were subjected to immunohistochemical and Western blot analyses for several markers of proliferation, apoptosis, and for rLOX-PP itself. Direct i.t. injection of rLOX-PP significantly reduced tumor volume on days 20, 22 and 25 and tumor weight at harvest on day 25 by 30% compared to control. Implantation of beads preloaded with 35 micrograms rLOX-PP (n = 10) in vivo reduced tumor volume and weight at sacrifice when compared to empty beads (p<0.05). A 30% reduction of tumor volume on days 22 and 25 (p<0.05) and final tumor weight on day 25 (p<0.05) were observed with a reduced tumor growth rate of 60% after implantation. rLOX-PP significantly reduced the expression of proliferation markers and Erk1/2 MAP kinase activation, while prominent increases in apoptosis markers were observed. rLOX-PP was detected by immunohistochemistry in harvested rLOX-PP tumors, but not in controls. Data provide pre-clinical findings that support proof of principle for the therapeutic anti-cancer potential of rLOX-PP protein formulations.
Conflict of interest statement
Figures








References
-
- Kagan HM, Trackman PC. Properties and function of lysyl oxidase. Am J Respir Cell Mol Biol. 1991;5:206–210. - PubMed
-
- Kessler E, Takahara K, Biniaminov L, Brusel M, Greenspan DS. Bone morphogenetic protein-1: the type I procollagen C-proteinase. Science. 1996;271:360–362. - PubMed
-
- Uzel MI, Scott IC, Babakhanlou-Chase H, Palamakumbura AH, Pappano WN, et al. Multiple bone morphogenetic protein 1-related mammalian metalloproteinases process pro-lysyl oxidase at the correct physiological site and control lysyl oxidase activation in mouse embryo fibroblast cultures. J Biol Chem. 2001;276:22537–22543. - PubMed
-
- Palamakumbura AH, Jeay S, Guo Y, Pischon N, Sommer P, et al. The propeptide domain of lysyl oxidase induces phenotypic reversion of ras-transformed cells. J Biol Chem. 2004;279:40593–40600. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

