Neonatal periostin knockout mice are protected from hyperoxia-induced alveolar simplication
- PMID: 22363622
- PMCID: PMC3281961
- DOI: 10.1371/journal.pone.0031336
Neonatal periostin knockout mice are protected from hyperoxia-induced alveolar simplication
Erratum in
-
Correction: Neonatal Periostin Knockout Mice Are Protected from Hyperoxia-Induced Alveolar Simplication.PLoS One. 2015 Jun 11;10(6):e0130369. doi: 10.1371/journal.pone.0130369. eCollection 2015. PLoS One. 2015. PMID: 26068377 Free PMC article. No abstract available.
Abstract
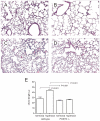
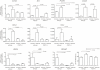
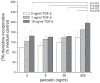
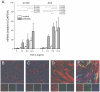
In bronchopulmonary dysplasia (BPD), alveolar septae are thickened with collagen and α-smooth muscle actin, transforming growth factor (TGF)-β-positive myofibroblasts. Periostin, a secreted extracellular matrix protein, is involved in TGF-β-mediated fibrosis and myofibroblast differentiation. We hypothesized that periostin expression is required for hypoalveolarization and interstitial fibrosis in hyperoxia-exposed neonatal mice, an animal model for this disease. We also examined periostin expression in neonatal lung mesenchymal stromal cells and lung tissue of hyperoxia-exposed neonatal mice and human infants with BPD. Two-to-three day-old wild-type and periostin null mice were exposed to air or 75% oxygen for 14 days. Mesenchymal stromal cells were isolated from tracheal aspirates of premature infants. Hyperoxic exposure of neonatal mice increased alveolar wall periostin expression, particularly in areas of interstitial thickening. Periostin co-localized with α-smooth muscle actin, suggesting synthesis by myofibroblasts. A similar pattern was found in lung sections of infants dying of BPD. Unlike wild-type mice, hyperoxia-exposed periostin null mice did not show larger air spaces or α-smooth muscle-positive myofibroblasts. Compared to hyperoxia-exposed wild-type mice, hyperoxia-exposed periostin null mice also showed reduced lung mRNA expression of α-smooth muscle actin, elastin, CXCL1, CXCL2 and CCL4. TGF-β treatment increased mesenchymal stromal cell periostin expression, and periostin treatment increased TGF-β-mediated DNA synthesis and myofibroblast differentiation. We conclude that periostin expression is increased in the lungs of hyperoxia-exposed neonatal mice and infants with BPD, and is required for hyperoxia-induced hypoalveolarization and interstitial fibrosis.
Conflict of interest statement
Figures





References
-
- Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2001;163:1723–1729. - PubMed
-
- Hussain NA, Siddiqui NH, Stocker JR. Pathology of arrested acinar development in postsurfactant bronchopulmonary dysplasia. Hum Pathol. 1998;29:710–717. - PubMed
-
- Coalson JJ. Pathology of new bronchopulmonary dysplasia. Semin Neonatol. 2003;8:73–81. - PubMed
-
- Toti P, Buonocore G, Tanganelli P, Catella AM, Palmeri ML, et al. Bronchopulmonary dysplasia of the premature baby: an immunohistochemical study. Pediatr Pulmonol. 1997;24:22–28. - PubMed
-
- Bhatt AJ, Pryhuber GS, Huyck H, Watkins RH, Metlay LA, et al. Disrupted pulmonary vasculature and decreased vascular endothelial growth factor, Flt-1, and TIE-2 in human infants dying with bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2001;164:1971–1980. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

