Propagation of epileptiform events across the corpus callosum in a cingulate cortical slice preparation
- PMID: 22363643
- PMCID: PMC3283628
- DOI: 10.1371/journal.pone.0031415
Propagation of epileptiform events across the corpus callosum in a cingulate cortical slice preparation
Abstract
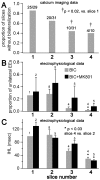
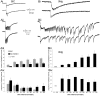
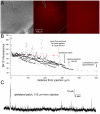
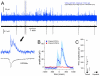
We report on a novel mouse in vitro brain slice preparation that contains intact callosal axons connecting anterior cingulate cortices (ACC). Callosal connections are demonstrated by the ability to regularly record epileptiform events between hemispheres (bilateral events). That the correlation of these events depends on the callosum is demonstrated by the bisection of the callosum in vitro. Epileptiform events are evoked with four different methods: (1) bath application of bicuculline (a GABA-A antagonist); (2) bicuculline+MK801 (an NMDA receptor antagonist), (3) a zero magnesium extracellular solution (0Mg); (4) focal application of bicuculline to a single cortical hemisphere. Significant increases in the number of epileptiform events, as well as increases in the ratio of bilateral events to unilateral events, are observed during bath applications of bicuculline, but not during applications of bicuculline+MK-801. Long ictal-like events (defined as events >20 seconds) are only observed in 0Mg. Whole cell patch clamp recordings of single neurons reveal strong feedforward inhibition during focal epileptiform events in the contralateral hemisphere. Within the ACC, we find differences between the rostral areas of ACC vs. caudal ACC in terms of connectivity between hemispheres, with the caudal regions demonstrating shorter interhemispheric latencies. The morphologies of many patch clamped neurons show callosally-spanning axons, again demonstrating intact callosal circuits in this in vitro preparation.
Conflict of interest statement
Figures



References
-
- Spencer SS. Corpus callosum section and other disconnection procedures for medically intractable epilepsy. Epilepsia. 1988;29(Suppl 2):S85–S99. - PubMed
-
- Spencer SS, Spencer DD, Sass K, Westerveld M, Katz A, et al. Anterior, total, and two-stage corpus callosum section: differential and incremental seizure responses. Epilepsia. 1993;34:561–567. - PubMed
-
- sadi-Pooya AA, Sharan A, Nei M, Sperling MR. Corpus callosotomy. Epilepsy Behav. 2008;13:271–278. - PubMed
-
- Marcus EM, Watson CW. Symmetrical epileptogenic foci in monkey cerebral cortex. Mechanisms of interaction and regional variations in capacity for synchronous discharges. Arch Neurol. 1968;19:99–116. - PubMed
-
- Erickson TC. Spread of the epileptic discharge - An experimental study of the after-discharge induced by electrical stimulation of the cerebral cortex. Archives of Neurology and Psychiatry. 1940;43:429–452.

