Effectiveness of terbutaline pump for the prevention of preterm birth. A systematic review and meta-analysis
- PMID: 22363704
- PMCID: PMC3283660
- DOI: 10.1371/journal.pone.0031679
Effectiveness of terbutaline pump for the prevention of preterm birth. A systematic review and meta-analysis
Abstract
Background: Subcutaneous terbutaline (SQ terbutaline) infusion by pump is used in pregnant women as a prolonged (beyond 48-72 h) maintenance tocolytic following acute treatment of preterm contractions. The effectiveness and safety of this maintenance tocolysis have not been clearly established. We aimed to systematically evaluate the effectiveness and safety of subcutaneous (SQ) terbutaline infusion by pump for maintenance tocolysis.
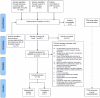
Methodology/principal findings: MEDLINE, EMBASE, CINAHL, the Cochrane Library, the Centre for Reviews and Dissemination databases, post-marketing surveillance data and grey literature were searched up to April 2011 for relevant experimental and observational studies. Two randomized trials, one nonrandomized trial, and 11 observational studies met inclusion criteria. Non-comparative studies were considered only for pump-related harms. We excluded case-reports but sought FDA summaries of post-marketing surveillance data. Non-English records without an English abstract were excluded. Evidence of low strength from observational studies with risk of bias favored SQ terbutaline pump for the outcomes of delivery at <32 and <37 weeks, mean days of pregnancy prolongation, and neonatal death. Observational studies of medium to high risk of bias also demonstrated benefit for other surrogate outcomes, such as birthweight and neonatal intensive care unit (NICU) admission. Several cases of maternal deaths and maternal cardiovascular events have been reported in patients receiving terbutaline tocolysis.
Conclusions/significance: Although evidence suggests that pump therapy may be beneficial as maintenance tocolysis, our confidence in its validity and reproducibility is low, suggesting that its use should be limited to the research setting. Concerns regarding safety of therapy persist.
Conflict of interest statement
Figures


References
-
- Behrman R, Butler A. Preterm Birth: Causes, Consequences, and Prevention. Washington (DC): National Academies Press; 2007. - PubMed
-
- Iams J, Romero R, Creasy R. Preterm labor and birth. In: Creasy R, Resnik R, Iams J, editors. Creasy and Resnik's Maternal-fetal medicine: principles and practice. Philadelphia PA: Saunders Elsevier; 2010. pp. 545–582.
-
- Hayes Inc. Continuous subcutaneous terbutaline infusion for treatment of preterm labor (Structured abstract). 2006. Health Technology Assessment Database John Wiley & Sons, Ltd.
-
- Owens DK, Lohr KN, Atkins D, Treadwell JR, Reston JT, et al. AHRQ series paper 5: grading the strength of a body of evidence when comparing medical interventions–agency for healthcare research and quality and the effective health-care program. J Clin Epidemiol. 2010;63:513–523. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

