Tachykinins stimulate a subset of mouse taste cells
- PMID: 22363709
- PMCID: PMC3283679
- DOI: 10.1371/journal.pone.0031697
Tachykinins stimulate a subset of mouse taste cells
Abstract
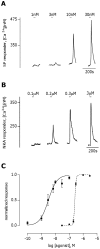
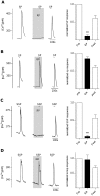
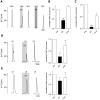
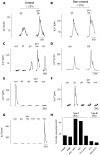
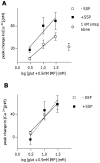
The tachykinins substance P (SP) and neurokinin A (NKA) are present in nociceptive sensory fibers expressing transient receptor potential cation channel, subfamily V, member 1 (TRPV1). These fibers are found extensively in and around the taste buds of several species. Tachykinins are released from nociceptive fibers by irritants such as capsaicin, the active compound found in chili peppers commonly associated with the sensation of spiciness. Using real-time Ca(2+)-imaging on isolated taste cells, it was observed that SP induces Ca(2+) -responses in a subset of taste cells at concentrations in the low nanomolar range. These responses were reversibly inhibited by blocking the SP receptor NK-1R. NKA also induced Ca(2+)-responses in a subset of taste cells, but only at concentrations in the high nanomolar range. These responses were only partially inhibited by blocking the NKA receptor NK-2R, and were also inhibited by blocking NK-1R indicating that NKA is only active in taste cells at concentrations that activate both receptors. In addition, it was determined that tachykinin signaling in taste cells requires Ca(2+)-release from endoplasmic reticulum stores. RT-PCR analysis further confirmed that mouse taste buds express NK-1R and NK-2R. Using Ca(2+)-imaging and single cell RT-PCR, it was determined that the majority of tachykinin-responsive taste cells were Type I (Glial-like) and umami-responsive Type II (Receptor) cells. Importantly, stimulating NK-1R had an additive effect on Ca(2+) responses evoked by umami stimuli in Type II (Receptor) cells. This data indicates that tachykinin release from nociceptive sensory fibers in and around taste buds may enhance umami and other taste modalities, providing a possible mechanism for the increased palatability of spicy foods.
Conflict of interest statement
Figures


Similar articles
-
Role of tachykinin and neurokinin receptors in the regulation of ovine omasal contractions.Regul Pept. 2012 Jan 10;173(1-3):64-73. doi: 10.1016/j.regpep.2011.09.007. Epub 2011 Oct 3. Regul Pept. 2012. PMID: 21971117
-
Tachykinins increase [3H]acetylcholine release in mouse striatum through multiple receptor subtypes.Neuroscience. 2000;95(2):367-76. doi: 10.1016/s0306-4522(99)00440-6. Neuroscience. 2000. PMID: 10658616
-
Effects of selective tachykinin-receptor antagonists on tachykinin-induced airway mucus secretion in the rat.Neuropeptides. 1999 Feb;33(1):55-61. doi: 10.1054/npep.1999.0014. Neuropeptides. 1999. PMID: 10657472
-
Tachykinins and tachykinin receptors in the gut, with special reference to NK2 receptors in human.Auton Neurosci. 2006 Jun 30;126-127:232-49. doi: 10.1016/j.autneu.2006.02.014. Epub 2006 Apr 17. Auton Neurosci. 2006. PMID: 16616700 Review.
-
Mechanisms of citric acid-induced bronchoconstriction.Am J Med. 2001 Dec 3;111 Suppl 8A:18S-24S. doi: 10.1016/s0002-9343(01)00816-6. Am J Med. 2001. PMID: 11749919 Review.
Cited by
-
Characterization and its implication of a novel taste receptor detecting nutrients in the honey bee, Apis mellifera.Sci Rep. 2019 Aug 12;9(1):11620. doi: 10.1038/s41598-019-46738-z. Sci Rep. 2019. PMID: 31406120 Free PMC article.
-
Substance P as a putative efferent transmitter mediates GABAergic inhibition in mouse taste buds.Br J Pharmacol. 2018 Apr;175(7):1039-1053. doi: 10.1111/bph.14142. Epub 2018 Feb 23. Br J Pharmacol. 2018. PMID: 29328505 Free PMC article.
-
Taste buds: cells, signals and synapses.Nat Rev Neurosci. 2017 Aug;18(8):485-497. doi: 10.1038/nrn.2017.68. Epub 2017 Jun 29. Nat Rev Neurosci. 2017. PMID: 28655883 Free PMC article. Review.
-
Fatigue, Stress, and Functional Status are Associated With Taste Changes in Oncology Patients Receiving Chemotherapy.J Pain Symptom Manage. 2021 Aug;62(2):373-382.e2. doi: 10.1016/j.jpainsymman.2020.11.029. Epub 2020 Nov 28. J Pain Symptom Manage. 2021. PMID: 33259906 Free PMC article.
-
Tachykinins and the potential causal factors for post-COVID-19 condition.Lancet Microbe. 2023 Aug;4(8):e642-e650. doi: 10.1016/S2666-5247(23)00111-8. Epub 2023 Jun 13. Lancet Microbe. 2023. PMID: 37327802 Free PMC article. Review.
References
-
- Caterina MJ, Julius D. The vanilloid receptor: a molecular gateway to the pain pathway. Annu Rev Neurosci. 2001;24:487–517. - PubMed
-
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, et al. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. - PubMed
-
- Pennefather JN, Lecci A, Candenas ML, Patak E, Pinto FM, et al. Tachykinins and tachykinin receptors: a growing family. Life Sci. 2004;74:1445–1463. - PubMed
-
- Moussaoui SM, Le Prado N, Bonici B, Faucher DC, Cuine F, et al. Distribution of neurokinin B in rat spinal cord and peripheral tissues: comparison with neurokinin A and substance P and effects of neonatal capsaicin treatment. Neuroscience. 1992;48:969–978. - PubMed
-
- Geppetti P, Nassini R, Materazzi S, Benemei S. The concept of neurogenic inflammation. BJU Int. 2008;101(Suppl 3):2–6. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

