The prevalence and cost of unapproved uses of top-selling orphan drugs
- PMID: 22363762
- PMCID: PMC3283698
- DOI: 10.1371/journal.pone.0031894
The prevalence and cost of unapproved uses of top-selling orphan drugs
Abstract
Introduction: The Orphan Drug Act encourages drug development for rare conditions. However, some orphan drugs become top sellers for unclear reasons. We sought to evaluate the extent and cost of approved and unapproved uses of orphan drugs with the highest unit sales.
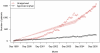
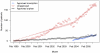
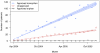
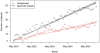
Methods: We assessed prescription patterns for four top-selling orphan drugs: lidocaine patch (Lidoderm) approved for post-herpetic neuralgia, modafinil (Provigil) approved for narcolepsy, cinacalcet (Sensipar) approved for hypercalcemia of parathyroid carcinoma, and imatinib (Gleevec) approved for chronic myelogenous leukemia and gastrointestinal stromal tumor. We pooled patient-specific diagnosis and prescription data from two large US state pharmaceutical benefit programs for the elderly. We analyzed the number of new and total patients using each drug and patterns of reimbursement for approved and unapproved uses. For lidocaine patch, we subcategorized approved prescriptions into two subtypes of unapproved uses: neuropathic pain, for which some evidence of efficacy exists, and non-neuropathic pain.
Results: We found that prescriptions for lidocaine patch, modafinil, and cinacalcet associated with non-orphan diagnoses rose at substantially higher rates (average monthly increases in number of patients of 14.6, 1.45, and 1.58) than prescriptions associated with their orphan diagnoses (3.12, 0.24, and 0.03, respectively (p<0.001 for all)). By contrast, for imatinib, approved uses increased significantly over off-label (0.97 vs. 0.47 patients, p<0.001). Spending on off-label uses was highest for lidocaine patch and modafinil (>75%). Increases in lidocaine patch use for non-neuropathic pain far exceeded neuropathic pain (10.2 vs. 3.6 patients, p<0.001).
Discussion: In our sample, three of four top-selling orphan drugs were used more commonly for non-orphan indications. These orphan drugs treated common clinical symptoms (pain and fatigue) or laboratory abnormalities. We should continue to monitor orphan drug use after approval to identify products that come to be widely used for non-FDA approved indications, particularly those without adequate evidence of efficacy.
Conflict of interest statement
Figures
Similar articles
-
Special FDA designations for drug development: orphan, fast track, accelerated approval, priority review, and breakthrough therapy.Eur J Health Econ. 2024 Aug;25(6):979-997. doi: 10.1007/s10198-023-01639-x. Epub 2023 Nov 14. Eur J Health Econ. 2024. PMID: 37962724 Free PMC article. Review.
-
Spending For Orphan Indications Among Top-Selling Orphan Drugs Approved To Treat Common Diseases.Health Aff (Millwood). 2021 Mar;40(3):453-460. doi: 10.1377/hlthaff.2020.01442. Health Aff (Millwood). 2021. PMID: 33646878 Free PMC article.
-
Beware the increasing cost and number of orphan drugs.Manag Care. 2013 Mar;22(3):10-4. Manag Care. 2013. PMID: 23610800 No abstract available.
-
What is wrong with orphan drug policies?Value Health. 2012 Dec;15(8):1185-91. doi: 10.1016/j.jval.2012.09.004. Value Health. 2012. PMID: 23244823
-
Approved and investigational uses of modafinil : an evidence-based review.Drugs. 2008;68(13):1803-39. doi: 10.2165/00003495-200868130-00003. Drugs. 2008. PMID: 18729534 Review.
Cited by
-
Special FDA designations for drug development: orphan, fast track, accelerated approval, priority review, and breakthrough therapy.Eur J Health Econ. 2024 Aug;25(6):979-997. doi: 10.1007/s10198-023-01639-x. Epub 2023 Nov 14. Eur J Health Econ. 2024. PMID: 37962724 Free PMC article. Review.
-
Outcomes of off-label drug uses in hospitals: a multicentric prospective study.Eur J Clin Pharmacol. 2014 Nov;70(11):1385-93. doi: 10.1007/s00228-014-1746-2. Epub 2014 Sep 9. Eur J Clin Pharmacol. 2014. PMID: 25196202 Free PMC article.
-
The European challenges of funding orphan medicinal products.Orphanet J Rare Dis. 2018 Nov 6;13(1):184. doi: 10.1186/s13023-018-0927-y. Orphanet J Rare Dis. 2018. PMID: 30396361 Free PMC article.
-
Personalizing health care: feasibility and future implications.BMC Med. 2013 Aug 13;11:179. doi: 10.1186/1741-7015-11-179. BMC Med. 2013. PMID: 23941275 Free PMC article. Review.
-
Pharmaceutical policy and innovation for rare diseases: A narrative review.F1000Res. 2023 Nov 13;12:211. doi: 10.12688/f1000research.130809.2. eCollection 2023. F1000Res. 2023. PMID: 38778810 Free PMC article. Review.
References
-
- Orphan Drug Act. 1983. Public Law Number 97-414 (96 Stat. 2049)
-
- Anand G. How drugs for rare diseases became lifeline for companies. 2005. Wall St. Journal 2005 Nov 15. - PubMed
-
- Wellman-Labadie O, Zhou Y. The US Orphan Drug Act: rare disease research stimulator or commercial opportunity? Health Policy. 2010;95:216–228. - PubMed
-
- Haffner ME, Torrent-Farnell J, Maher PD. Does orphan drug legislation really answer the needs of patients? Lancet. 2008;371:2041–2044. - PubMed
-
- Rohde DD. The Orphan Drug Act: an engine of innovation? At what cost? Food Drug Law J. 2000;55:125–143. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

