Circulating sCD14 is associated with virological response to pegylated-interferon-alpha/ribavirin treatment in HIV/HCV co-infected patients
- PMID: 22363790
- PMCID: PMC3283684
- DOI: 10.1371/journal.pone.0032028
Circulating sCD14 is associated with virological response to pegylated-interferon-alpha/ribavirin treatment in HIV/HCV co-infected patients
Abstract
Objectives: Microbial translocation (MT) through the gut accounts for immune activation and CD4+ loss in HIV and may influence HCV disease progression in HIV/HCV co-infection. We asked whether increased MT and immune activation may hamper anti-HCV response in HIV/HCV patients.
Methods: 98 HIV/HCV patients who received pegylated-alpha-interferon (peg-INF-alpha)/ribavirin were retrospectively analyzed. Baseline MT (lipopolysaccharide, LPS), host response to MT (sCD14), CD38+HLA-DR+CD4+/CD8+, HCV genotype, severity of liver disease were assessed according to Early Virological Response (EVR: HCV-RNA <50 IU/mL at week 12 of therapy or ≥2 log(10) reduction from baseline after 12 weeks of therapy) and Sustained Virological Response (SVR: HCV-RNA <50 IU/mL 24 weeks after end of therapy). Mann-Whitney/Chi-square test and Pearson's correlation were used. Multivariable regression was performed to determine factors associated with EVR/SVR.
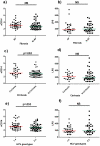
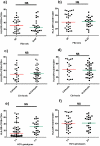
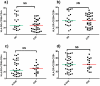
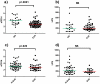
Results: 71 patients displayed EVR; 41 SVR. Patients with HCV genotypes 1-4 and cirrhosis presented a trend to higher sCD14, compared to patients with genotypes 2-3 (p = 0.053) and no cirrhosis (p = 0.052). EVR and SVR patients showed lower levels of circulating sCD14 (p = 0.0001, p = 0.026, respectively), but similar T-cell activation compared to Non-EVR (Null Responders, NR) and Non-SVR (N-SVR) subjects. sCD14 resulted the main predictive factor of EVR (0.145 for each sCD14 unit more, 95%CI 0.031-0.688, p = 0.015). SVR was associated only with HCV genotypes 2-3 (AOR 0.022 for genotypes 1-4 vs 2-3, 95%CI 0.001-0.469, p = 0.014).
Conclusions: In HIV/HCV patients sCD14 correlates with the severity of liver disease and predicts early response to peg-INF-alpha/ribavirin, suggesting MT-driven immune activation as pathway of HIV/HCV co-infection and response to therapy.
Conflict of interest statement
Figures
Similar articles
-
Low levels of microbial translocation marker LBP are associated with sustained viral response after anti-HCV treatment in HIV-1/HCV co-infected patients.PLoS One. 2015 Mar 18;10(3):e0118643. doi: 10.1371/journal.pone.0118643. eCollection 2015. PLoS One. 2015. PMID: 25785448 Free PMC article.
-
Predictive value of rapid virological response and early virological response on sustained virological response in HCV patients treated with pegylated interferon alpha-2a and ribavirin.J Gastroenterol Hepatol. 2007 Jun;22(6):832-6. doi: 10.1111/j.1440-1746.2007.04904.x. J Gastroenterol Hepatol. 2007. PMID: 17565637
-
Response to pegylated interferon plus ribavirin in HIV-infected patients with chronic hepatitis C due to genotype 4.J Viral Hepat. 2008 Oct;15(10):710-5. doi: 10.1111/j.1365-2893.2008.01015.x. Epub 2008 Jul 10. J Viral Hepat. 2008. PMID: 18637070 Clinical Trial.
-
Financial impact of two different ways of evaluating early virological response to peginterferon-alpha-2b plus ribavirin therapy in treatment-naive patients with chronic hepatitis C virus genotype 1.Pharmacoeconomics. 2005;23(10):1043-55. doi: 10.2165/00019053-200523100-00007. Pharmacoeconomics. 2005. PMID: 16235977 Review.
-
Variable Normalization of Naïve CD4+ Lymphopenia and Markers of Monocyte and T Cell Activation over the Course of Direct-Acting Anti-Viral Treatment of Chronic Hepatitis C Virus Infection.Viruses. 2021 Dec 29;14(1):50. doi: 10.3390/v14010050. Viruses. 2021. PMID: 35062255 Free PMC article. Review.
Cited by
-
Low levels of microbial translocation marker LBP are associated with sustained viral response after anti-HCV treatment in HIV-1/HCV co-infected patients.PLoS One. 2015 Mar 18;10(3):e0118643. doi: 10.1371/journal.pone.0118643. eCollection 2015. PLoS One. 2015. PMID: 25785448 Free PMC article.
-
Chronic Hepatitis C Virus Infection and the Proinflammatory Effects of Injection Drug Use.J Infect Dis. 2016 Nov 1;214(9):1376-1382. doi: 10.1093/infdis/jiw373. Epub 2016 Aug 11. J Infect Dis. 2016. PMID: 27521361 Free PMC article. Clinical Trial.
-
Cellular activation and intracellular HCV load in peripheral blood monocytes isolated from HCV monoinfected and HIV-HCV coinfected patients.PLoS One. 2014 May 8;9(5):e96907. doi: 10.1371/journal.pone.0096907. eCollection 2014. PLoS One. 2014. PMID: 24809719 Free PMC article.
-
Brief Report: CD14brightCD16- monocytes and sCD14 level negatively associate with CD4-memory T-cell frequency and predict HCV-decline on therapy.J Acquir Immune Defic Syndr. 2016 Nov 1;73(3):258-262. doi: 10.1097/QAI.0000000000001104. J Acquir Immune Defic Syndr. 2016. PMID: 27258231 Free PMC article. Clinical Trial.
-
Microbial translocation in chronic liver diseases.Int J Microbiol. 2012;2012:694629. doi: 10.1155/2012/694629. Epub 2012 Jul 17. Int J Microbiol. 2012. PMID: 22848224 Free PMC article.
References
-
- Rockstroh JK, Mocroft A, Soriano V, Tural C, Losso MH, et al. Influence of hepatitis C virus infection on HIV-1 disease progression and response to highly active antiretroviral therapy. J Infect Dis. 2005;192:992–1002. - PubMed
-
- Soriano V, Mocroft A, Rockstroh J, Ledergerber B, Knysz B, et al. Spontaneous viral clearance, viral load, and genotype distribution of hepatitis C virus (HCV) in HIV-infected patients with anti-HCV antibodies in Europe. J Infect Dis. 2008;198:1337–1344. - PubMed
-
- Bica I, McGovern B, Dhar R, Stone D, McGowan K, et al. Increasing mortality due to end-stage liver disease in patients with human immunodeficiency virus infection. Clin Infect Dis. 2001;32:492–497. - PubMed
-
- Graham CS, Baden LR, Yu E, Mrus JM, Carnie J, et al. Influence of human immunodeficiency virus infection on the course of hepatitis C virus infection: a meta-analysis. Clin Infect Dis. 2001;33:562–569. - PubMed
-
- Rosenthal E, Salmon-Céron D, Lewden C, Bouteloup V, Pialoux G, et al. Liver-related deaths in HIV-infected patients between 1995 and 2005 in the French GERMIVIC Joint Study Group Network (Mortavic 2005 study in collaboration with the Mortalité 2005 survey, ANRS EN19). HIV Med. 2009;10:282–289. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

