Cell signalling by reactive lipid species: new concepts and molecular mechanisms
- PMID: 22364280
- PMCID: PMC3286857
- DOI: 10.1042/BJ20111752
Cell signalling by reactive lipid species: new concepts and molecular mechanisms
Abstract
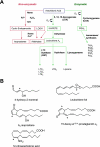
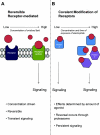
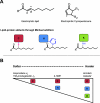
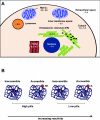
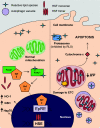
The process of lipid peroxidation is widespread in biology and is mediated through both enzymatic and non-enzymatic pathways. A significant proportion of the oxidized lipid products are electrophilic in nature, the RLS (reactive lipid species), and react with cellular nucleophiles such as the amino acids cysteine, lysine and histidine. Cell signalling by electrophiles appears to be limited to the modification of cysteine residues in proteins, whereas non-specific toxic effects involve modification of other nucleophiles. RLS have been found to participate in several physiological pathways including resolution of inflammation, cell death and induction of cellular antioxidants through the modification of specific signalling proteins. The covalent modification of proteins endows some unique features to this signalling mechanism which we have termed the 'covalent advantage'. For example, covalent modification of signalling proteins allows for the accumulation of a signal over time. The activation of cell signalling pathways by electrophiles is hierarchical and depends on a complex interaction of factors such as the intrinsic chemical reactivity of the electrophile, the intracellular domain to which it is exposed and steric factors. This introduces the concept of electrophilic signalling domains in which the production of the lipid electrophile is in close proximity to the thiol-containing signalling protein. In addition, we propose that the role of glutathione and associated enzymes is to insulate the signalling domain from uncontrolled electrophilic stress. The persistence of the signal is in turn regulated by the proteasomal pathway which may itself be subject to redox regulation by RLS. Cell death mediated by RLS is associated with bioenergetic dysfunction, and the damaged proteins are probably removed by the lysosome-autophagy pathway.
Figures

References
-
- Pratico D., Lawson J. A., Rokach J., FitzGerald G. A. The isoprostanes in biology and medicine. Trends Endocrinol. Metab. 2001;12:243–247. - PubMed
-
- Gaut J. P., Heinecke J. W. Mechanisms for oxidizing low-density lipoprotein. Insights from patterns of oxidation products in the artery wall and from mouse models of atherosclerosis. Trends Cardiovasc. Med. 2001;11:103–112. - PubMed
-
- Milne G. L., Musiek E. S., Morrow J. D. F2-isoprostanes as markers of oxidative stress in vivo: an overview. Biomarkers. 2005;10:S10–S23. - PubMed
-
- Marwah S. S., Blann A. D., Rea C., Phillips J. D., Wright J., Bareford D. Reduced vitamin E antioxidant capacity in sickle cell disease is related to transfusion status but not to sickle crisis. Am. J. Hematol. 2002;69:144–146. - PubMed
-
- Yin H., Porter N. A. New insights regarding the autoxidation of polyunsaturated fatty acids. Antioxid. Redox Signaling. 2005;7:170–184. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

