Effects of dexpramipexole on brain mitochondrial conductances and cellular bioenergetic efficiency
- PMID: 22364637
- PMCID: PMC3746080
- DOI: 10.1016/j.brainres.2012.01.046
Effects of dexpramipexole on brain mitochondrial conductances and cellular bioenergetic efficiency
Abstract
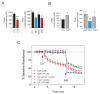
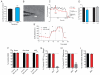
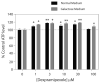
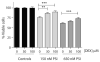
Cellular stress or injury can result in mitochondrial dysfunction, which has been linked to many chronic neurological disorders including amyotrophic lateral sclerosis (ALS) and Parkinson's disease (PD). Stressed and dysfunctional mitochondria exhibit an increase in large conductance mitochondrial membrane currents and a decrease in bioenergetic efficiency. Inefficient energy production puts cells, and particularly neurons, at risk of death when energy demands exceed cellular energy production. Here we show that the candidate ALS drug dexpramipexole (DEX; KNS-760704; ((6R)-4,5,6,7-tetrahydro-N6-propyl-2,6-benzothiazole-diamine) and cyclosporine A (CSA) inhibited increases in ion conductance in whole rat brain-derived mitochondria induced by calcium or treatment with a proteasome inhibitor, although only CSA inhibited calcium-induced permeability transition in liver-derived mitochondria. In several cell lines, including cortical neurons in culture, DEX significantly decreased oxygen consumption while maintaining or increasing production of adenosine triphosphate (ATP). DEX also normalized the metabolic profile of injured cells and was protective against the cytotoxic effects of proteasome inhibition. These data indicate that DEX increases the efficiency of oxidative phosphorylation, possibly by inhibition of a CSA-sensitive mitochondrial conductance.
Copyright © 2012 Elsevier B.V. All rights reserved.
Figures


References
-
- Abramova NA, Cassarino DS, Khan SM, Painter TW, Bennett JP., Jr. Inhibition by R(+) or S(−) pramipexole of caspase activation and cell death induced by methylpyridinium ion or beta amyloid peptide in SH-SY5Y neuroblastoma. Journal of Neuroscience Research. 2002;67:494–500. - PubMed
-
- Alavian L, Collis, Bonanni, Zeng, Sacchetti, Lazrove, Nabili, Flaherty, Graham, Chen, Messerli, Mariggio, Rahner, McNay, Shore, Smith, Hardwick, Jonas Bcl-x(L) regulates metabolic efficiency of neurons through interaction with the mitochondrial F(1)F(O) ATP synthase. Nat Cell Biol. 2011;13:1224–33. - PMC - PubMed
-
- Andrews ZB, Diano S, Horvath TL. Mitochondrial uncoupling proteins in the CNS: in support of function and survival. Nature Reviews Neuroscience. 2005;6:829–40. - PubMed
-
- Bandopadhyay R, de Belleroche J. Pathogenesis of Parkinson’s disease: emerging role of molecular chaperones. Trends in Molecular Medicine. 2010;16:27–36. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

