Characterization of bovine glucose transporter 1 kinetics and substrate specificities in Xenopus oocytes
- PMID: 22365203
- PMCID: PMC4337999
- DOI: 10.3168/jds.2011-4430
Characterization of bovine glucose transporter 1 kinetics and substrate specificities in Xenopus oocytes
Abstract
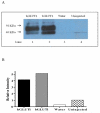
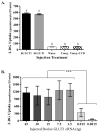
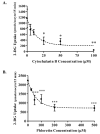
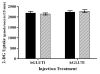
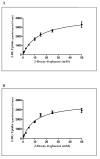
Glucose is an essential substrate for lactose synthesis and an important energy source in milk production. Glucose uptake in the mammary gland, therefore, plays a critical role in milk synthesis. Facilitative glucose transporters (GLUT) mediate glucose uptake in the mammary gland. Glucose transporter 1 (GLUT1) is the major facilitative glucose transporter expressed in the bovine mammary gland and has been shown to localize to the basolateral membrane of mammary epithelial cells. Glucose transporter 1 is, therefore, thought to play a major role in glucose uptake during lactation. The objective of this study was to determine the transport kinetic properties and substrate specificity of bovine GLUT1 using the Xenopus oocyte model. Bovine GLUT1 (bGLUT1) was expressed in Xenopus oocytes by microinjection of in vitro transcribed cRNA and was found to be localized to the plasma membrane, which resulted in increased glucose uptake. This bGLUT1-mediated glucose uptake was dramatically inhibited by specific facilitative glucose transport inhibitors, cytochalasin B, and phloretin. Kinetic analysis of bovine and human GLUT1 was conducted under zero-trans conditions using radio-labeled 2-deoxy-D-glucose and the principles of Michaelis-Menten kinetics. Bovine GLUT1 exhibited a Michaelis constant (K(m)) of 9.8 ± 3.0mM for 2-deoxy-d-glucose, similar to 11.7 ± 3.7 mM for human GLUT1. Transport by bGLUT1 was inhibited by mannose and galactose, but not fructose, indicating that bGLUT1 may also be able to transport mannose and galactose. Our data provides functional insight into the transport properties of bGLUT1 in taking up glucose across mammary epithelial cells for milk synthesis.
Copyright © 2012 American Dairy Science Association. Published by Elsevier Inc. All rights reserved.
Figures


References
-
- Ahmed N, Berridge MV. N-glycosylation of glucose transporter-1 (Glut-1) is associated with increased transporter affinity for glucose in human leukemic cells. Leuk. Res. 1999;23:395–401. - PubMed
-
- Asano T, Katagiri H, Takata K, Lin JL, Ishihara H, Inukai K, Tsukuda K, Kikuchi M, Hirano H, Yazaki Y, et al. The role of N-glycosylation of GLUT1 for glucose transport activity. J. Biol. Chem. 1991;266:24632–24636. - PubMed
-
- Burant CF, Bell GI. Mammalian facilitative glucose transporters: evidence for similar substrate recognition sites in functionally monomeric proteins. Biochemistry. 1992;31:10414–10420. - PubMed
-
- Corpe CP, Bovelander FJ, Munoz CM, Hoekstra JH, Simpson IA, Kwon O, Levine M, Burant CF. Cloning and functional characterization of the mouse fructose transporter, GLUT5. Biochim. Biophys. Acta. 2002;1576:191–197. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

