Cholesterol catabolism by Mycobacterium tuberculosis requires transcriptional and metabolic adaptations
- PMID: 22365605
- PMCID: PMC3292763
- DOI: 10.1016/j.chembiol.2011.12.016
Cholesterol catabolism by Mycobacterium tuberculosis requires transcriptional and metabolic adaptations
Erratum in
- Chem Biol. 2012 Sep 21;19(9):1210
Abstract
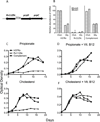
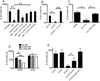
To understand the adaptation of Mycobacterium tuberculosis to the intracellular environment, we used comprehensive metabolite profiling to identify the biochemical pathways utilized during growth on cholesterol, a critical carbon source during chronic infection. Metabolic alterations observed during cholesterol catabolism centered on propionyl-CoA and pyruvate pools. Consequently, growth on this substrate required the transcriptional induction of the propionyl-CoA-assimilating methylcitrate cycle (MCC) enzymes, via the Rv1129c regulatory protein. We show that both Rv1129c and the MCC enzymes are required for intracellular growth in macrophages and that the growth defect of MCC mutants is largely attributable to the degradation of host-derived cholesterol. Together, these observations define a coordinated transcriptional and metabolic adaptation that is required for scavenging carbon during intracellular growth.
Copyright © 2012 Elsevier Ltd. All rights reserved.
Figures



References
-
- Appelberg R. Macrophage nutriprive antimicrobial mechanisms. J Leukoc Biol. 2006;79:1117–1128. - PubMed
-
- Brock M, Buckel W. On the mechanism of action of the antifungal agent propionate. Eur J Biochem. 2004;271:3227–3241. - PubMed
-
- Chang JC, Harik NS, Liao RP, Sherman DR. Identification of Mycobacterial genes that alter growth and pathology in macrophages and in mice. J Infect Dis. 2007;196:788–795. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

