Constitutive type I interferon modulates homeostatic balance through tonic signaling
- PMID: 22365663
- PMCID: PMC3294371
- DOI: 10.1016/j.immuni.2012.01.011
Constitutive type I interferon modulates homeostatic balance through tonic signaling
Abstract
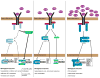
Interferons (IFNs) were discovered as cytokines induced during and protecting from viral infection. They have been documented to play essential roles in numerous physiological processes beyond antiviral and antimicrobial defense, including immunomodulation, cell cycle regulation, cell survival, and cell differentiation. Recent data have also uncovered a potentially darker side to IFN, including roles in inflammatory diseases, such as autoimmunity and diabetes. IFN can have effects in the absence of acute infection, highlighting a physiologic role for constitutive IFN. Type I IFNs are constitutively produced at vanishingly low quantities and yet exert profound effects, mediated in part through modulation of signaling intermediates required for responses to diverse cytokines. We review evidence for a yin-yang of IFN function through its role in modulating crosstalk between multiple cytokines by both feedforward and feedback regulation of common signaling intermediates and postulate a homeostatic role for IFN through tonic signaling in the absence of acute infection.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures

References
-
- Arakura F, Hida S, Ichikawa E, Yajima C, Nakajima S, Saida T, Taki S. Genetic control directed toward spontaneous IFN-alpha/IFN-beta responses and downstream IFN-gamma expression influences the pathogenesis of a murine psoriasis-like skin disease. J Immunol. 2007;179:3249–3257. - PubMed
-
- Bach EA, Aguet M, Schreiber RD. The IFN gamma receptor: a paradigm for cytokine receptor signaling. Annu Rev Immunol. 1997;15:563–591. - PubMed
-
- Billard C, Sigaux F, Castaigne S, Valensi F, Flandrin G, Degos L, Falcoff E, Aguet M. Treatment of hairy cell leukemia with recombinant alpha interferon: II. In vivo down-regulation of alpha interferon receptors on tumor cells. Blood. 1986;67:821–826. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

