Polyethyleneimine-lipid conjugate-based pH-sensitive micellar carrier for gene delivery
- PMID: 22365809
- PMCID: PMC3527089
- DOI: 10.1016/j.biomaterials.2011.11.088
Polyethyleneimine-lipid conjugate-based pH-sensitive micellar carrier for gene delivery
Abstract
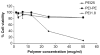
A low molecular weight polyethyleneimine (PEI 1.8 kDa) was modified with dioleoylphosphatidylethanolamine (PE) to form the PEI-PE conjugate investigated as a transfection vector. The optimized PEI-PE/pDNA complexes at an N/P ratio of 16 had a particle size of 225 nm, a surface charge of +31 mV, and protected the pDNA from the action of DNase I. The PEI-PE conjugate had a critical micelle concentration (CMC) of about 34 μg/ml and exhibited no toxicity compared to a high molecular weight PEI (PEI 25 kDa) as tested with B16-F10 melanoma cells. The B16-F10 cells transfected with PEI-PE/pEGFP complexes showed protein expression levels higher than with PEI-1.8 or PEI-25 vectors. Complexes prepared with YOYO 1-labeled pEGFP confirmed the enhanced delivery of the plasmid with PEI-PE compared to PEI-1.8 and PEI-25. The PEI-PE/pDNA complexes were also mixed with various amounts of micelle-forming material, polyethylene glycol (PEG)-PE to improve biocompatibility. The resulting particles exhibited a neutral surface charge, resistance to salt-induced aggregation, and good transfection activity in the presence of serum in complete media. The use of the low-pH-degradable PEG-hydrazone-PE produced particles with transfection activity sensitive to changes in pH consistent with the relatively acidic tumor environment.
Copyright © 2012 Elsevier Ltd. All rights reserved.
Figures





References
-
- Akinc A, Thomas M, Klibanov AM, Langer R. Exploring polyethylenimine-mediated DNA transfection and the proton sponge hypothesis. J Gene Med. 2005;7:657–63. - PubMed
-
- Sonawane ND, Szoka FC, Jr, Verkman AS. Chloride accumulation and swelling in endosomes enhances DNA transfer by polyamine-DNA polyplexes. J Biol Chem. 2003;278:44826–31. - PubMed
-
- Brunner S, Furtbauer E, Sauer T, Kursa M, Wagner E. Overcoming the nuclear barrier: cell cycle independent nonviral gene transfer with linear poly-ethylenimine or electroporation. Mol Ther. 2002;5:80–6. - PubMed
-
- Kircheis R, Wightman L, Wagner E. Design and gene delivery activity of modified polyethylenimines. Adv Drug Deliv Rev. 2001;53:341–58. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

