Young dentate granule cells mediate pattern separation, whereas old granule cells facilitate pattern completion
- PMID: 22365813
- PMCID: PMC3319279
- DOI: 10.1016/j.cell.2012.01.046
Young dentate granule cells mediate pattern separation, whereas old granule cells facilitate pattern completion
Abstract
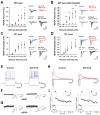
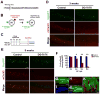
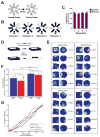
Adult-born granule cells (GCs), a minor population of cells in the hippocampal dentate gyrus, are highly active during the first few weeks after functional integration into the neuronal network, distinguishing them from less active, older adult-born GCs and the major population of dentate GCs generated developmentally. To ascertain whether young and old GCs perform distinct memory functions, we created a transgenic mouse in which output of old GCs was specifically inhibited while leaving a substantial portion of young GCs intact. These mice exhibited enhanced or normal pattern separation between similar contexts, which was reduced following ablation of young GCs. Furthermore, these mutant mice exhibited deficits in rapid pattern completion. Therefore, pattern separation requires adult-born young GCs but not old GCs, and older GCs contribute to the rapid recall by pattern completion. Our data suggest that as adult-born GCs age, their function switches from pattern separation to rapid pattern completion.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures




Comment in
-
Learning and memory: adult-born neurons change jobs.Nat Rev Neurosci. 2012 Mar 20;13(4):219. doi: 10.1038/nrn3227. Nat Rev Neurosci. 2012. PMID: 22430015 No abstract available.
-
The virtues of youth and maturity (in dentate granule cells).Cell. 2012 Mar 30;149(1):18-20. doi: 10.1016/j.cell.2012.02.038. Cell. 2012. PMID: 22464319
References
-
- Altman J, Bayer SA. Migration and distribution of two populations of hippocampal granule cell precursors during the perinatal and postnatal periods. J Comp Neurol. 1990;301:365–381. - PubMed
-
- Altman J, Das GD. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J Comp Neurol. 1965;124:319–335. - PubMed
-
- Amaral DG, Ishizuka N, Claiborne B. Neurons, numbers and the hippocampal network. Prog Brain Res. 1990;83:1–11. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

