Implementation of the Department of Veterans Affairs' first point-of-care clinical trial
- PMID: 22366293
- PMCID: PMC3392864
- DOI: 10.1136/amiajnl-2011-000623
Implementation of the Department of Veterans Affairs' first point-of-care clinical trial
Abstract
Objectives: The Massachusetts Veterans Epidemiology Research and Information Center in collaboration with the Stanford Center for Innovative Study Design set out to test the feasibility of a new method of evidence generation. The first pilot of a point-of-care clinical trial (POCCT), adding randomization and other study processes to an electronic medical record (EMR) system, was launched to compare the effectiveness of two insulin regimens.
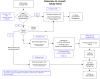
Materials and methods: Existing functionalities of the Veterans Affairs (VA) computerized patient record system (CPRS)/veterans health information systems and technology architecture (VISTA) were modified to support the activities of a randomized controlled trial including enrolment, randomization, and longitudinal data collection.
Results: The VA's CPRS/VISTA was successfully adapted to support the processes of a clinical trial and longitudinal study data are being collected from the medical record automatically. As of 30 June 2011, 55 of the 67 eligible patients approached received a randomized intervention.
Discussion: The design of CPRS/VISTA made integration of study workflows and data collection possible. Institutions and investigators considering similar designs must carefully map clinical workflows and clinical trial workflows to EMR capabilities. POCCT study teams are necessarily interdisciplinary and interdepartmental. As a result, executive sponsorship is critical.
Conclusion: POCCT represent a promising new method for conducting clinical science. Much work is needed to understand better the optimal uses and designs for this new approach. Next steps include focus groups to measure patient and clinician perceptions, multisite deployment of the current pilot, and implementation of additional studies.
Conflict of interest statement
Figures

References
-
- Institute of Medicine Learning What Works Best; The Nation's Need for Evidence on Comparative Effectiveness in Health Care. Washington DC, USA: Institute of Medicine of the National Academies, 2007. http://www.iom.edu/ebm-effectiveness
-
- Institute of Medicine Initial National Priorities for Comparative Effectiveness Research. Washington DC, USA: Institute of Medicine of the National Academies, 2009. http://www.iom.edu/ebm-effectiveness
-
- Congressional Budget Office Research on the Comparative Effectiveness of Medical Treatments: Issues and Options for an Expanded Federal Role. Washington, DC, USA: Congress of the United States, Congressional Budget Office, 2007
-
- Federal Coordinating Council for Comparative Effectiveness Research Report to the President and the Congress: U.S. Department of Health and Human Services. Washington DC, USA: US Department of Health and Human Services, 2009
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

