Met receptor tyrosine kinase signals through a cortactin-Gab1 scaffold complex, to mediate invadopodia
- PMID: 22366451
- PMCID: PMC3434810
- DOI: 10.1242/jcs.100834
Met receptor tyrosine kinase signals through a cortactin-Gab1 scaffold complex, to mediate invadopodia
Abstract
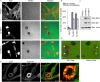
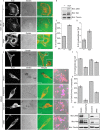
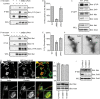
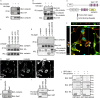
Invasive carcinoma cells form actin-rich matrix-degrading protrusions called invadopodia. These structures resemble podosomes produced by some normal cells and play a crucial role in extracellular matrix remodeling. In cancer, formation of invadopodia is strongly associated with invasive potential. Although deregulated signals from the receptor tyrosine kinase Met (also known as hepatocyte growth factor are linked to cancer metastasis and poor prognosis, its role in invadopodia formation is not known. Here we show that stimulation of breast cancer cells with the ligand for Met, hepatocyte growth factor, promotes invadopodia formation, and in aggressive gastric tumor cells where Met is amplified, invadopodia formation is dependent on Met activity. Using both GRB2-associated-binding protein 1 (Gab1)-null fibroblasts and specific knockdown of Gab1 in tumor cells we show that Met-mediated invadopodia formation and cell invasion requires the scaffold protein Gab1. By a structure-function approach, we demonstrate that two proline-rich motifs (P4/5) within Gab1 are essential for invadopodia formation. We identify the actin regulatory protein, cortactin, as a direct interaction partner for Gab1 and show that a Gab1-cortactin interaction is dependent on the SH3 domain of cortactin and the integrity of the P4/5 region of Gab1. Both cortactin and Gab1 localize to invadopodia rosettes in Met-transformed cells and the specific uncoupling of cortactin from Gab1 abrogates invadopodia biogenesis and cell invasion downstream from the Met receptor tyrosine kinase. Met localizes to invadopodia along with cortactin and promotes phosphorylation of cortactin. These findings provide insights into the molecular mechanisms of invadopodia formation and identify Gab1 as a scaffold protein involved in this process.
Figures




References
-
- Artym V. V., Zhang Y., Seillier–Moiseiwitsch F., Yamada K. M., Mueller S. C. (2006). Dynamic interactions of cortactin and membrane type 1 matrix metalloproteinase at invadopodia: defining the stages of invadopodia formation and function. Cancer Res. 66, 3034–3043 10.1158/0008-5472.CAN-05-2177 - DOI - PubMed
-
- Ayala I., Giacchetti G., Caldieri G., Attanasio F., Mariggiò S., Tetè S., Polishchuk R., Castronovo V., Buccione R. (2009). Faciogenital dysplasia protein Fgd1 regulates invadopodia biogenesis and extracellular matrix degradation and is up-regulated in prostate and breast cancer. Cancer Res. 69, 747–752 10.1158/0008-5472.CAN-08-1980 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

