Regulation of nuclear PKA revealed by spatiotemporal manipulation of cyclic AMP
- PMID: 22366721
- PMCID: PMC3307945
- DOI: 10.1038/nchembio.799
Regulation of nuclear PKA revealed by spatiotemporal manipulation of cyclic AMP
Erratum in
- Nat Chem Biol. 2013 Jun;9(6):406
Abstract
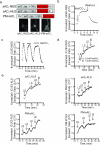
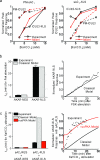
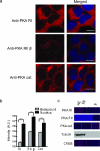
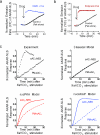
Understanding how specific cyclic AMP (cAMP) signals are organized and relayed to their effectors in different compartments of the cell to achieve functional specificity requires molecular tools that allow precise manipulation of cAMP in these compartments. Here we characterize a new method using bicarbonate-activatable and genetically targetable soluble adenylyl cyclase to control the location, kinetics and magnitude of the cAMP signal. Using this live-cell cAMP manipulation in conjunction with fluorescence imaging and mechanistic modeling, we uncovered the activation of a resident pool of protein kinase A (PKA) holoenzyme in the nuclei of HEK-293 cells, modifying the existing dogma of cAMP-PKA signaling in the nucleus. Furthermore, we show that phosphodiesterases and A-kinase anchoring proteins (AKAPs) are critical in shaping nuclear PKA responses. Collectively, our data suggest a new model in which AKAP-localized phosphodiesterases tune an activation threshold for nuclear PKA holoenzyme, thereby converting spatially distinct second messenger signals to temporally controlled nuclear kinase activity.
Figures


References
-
- Tasken K, Aandahl EM. Localized effects of cAMP mediated by distinct routes of protein kinase A. Physiol Rev. 2004;84:137–67. - PubMed
-
- Steinberg SF, Brunton LL. Compartmentation of G protein-coupled signaling pathways in cardiac myocytes. Annual Review of Pharmacology and Toxicology. 2001;41:751–773. - PubMed
-
- Houslay MD. Underpinning compartmentalised cAMP signalling through targeted cAMP breakdown. Trends Biochem Sci. 2010;35:91–100. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

