An APC/C inhibitor stabilizes cyclin B1 by prematurely terminating ubiquitination
- PMID: 22366722
- PMCID: PMC3307893
- DOI: 10.1038/nchembio.801
An APC/C inhibitor stabilizes cyclin B1 by prematurely terminating ubiquitination
Abstract
The anaphase-promoting complex/cyclosome (APC) is a ubiquitin ligase that is required for exit from mitosis. We previously showed that tosyl arginine methyl ester (TAME) inhibits APC-dependent proteolysis by competing with the C-terminal isoleucine-arginine tail of the APC activator cell division cycle 20 (Cdc20) for APC binding. Here we show that in the absence of APC substrates, TAME ejects Cdc20 from the APC by promoting Cdc20 autoubiquitination in its N-terminal region. Cyclin B1 antagonizes TAME's effect by promoting binding of free Cdc20 to the APC and by suppressing Cdc20 autoubiquitination. Nevertheless, TAME stabilizes cyclin B1 in Xenopus extracts by two mechanisms. First, it reduces the k(cat) of the APC-Cdc20-cyclin B1 complex without affecting the K(m), slowing the initial ubiquitination of unmodified cyclin B1. Second, as cyclin B1 becomes ubiquitinated, it loses its ability to promote Cdc20 binding to the APC in the presence of TAME. As a result, cyclin B1 ubiquitination terminates before reaching the threshold necessary for proteolysis.
Figures
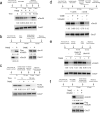

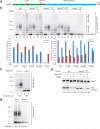
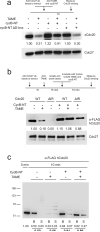
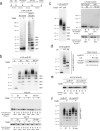

Comment in
-
Ubiquitin ligases: Taming the APC.Nat Chem Biol. 2012 Mar 16;8(4):323-4. doi: 10.1038/nchembio.923. Nat Chem Biol. 2012. PMID: 22426190 No abstract available.
References
-
- Peters JM. The anaphase promoting complex/cyclosome: a machine designed to destroy. Nat Rev Mol Cell Biol. 2006;7:644–656. - PubMed
-
- Verma R, et al. Ubistatins inhibit proteasome-dependent degradation by binding the ubiquitin chain. Science. 2004;306:117–120. - PubMed
-
- Hayes MJ, et al. Early mitotic degradation of Nek2A depends on Cdc20-independent interaction with the APC/C. Nat Cell Biol. 2006;8:607–614. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

