Serotonergic transcriptional networks and potential importance to mental health
- PMID: 22366757
- PMCID: PMC3594782
- DOI: 10.1038/nn.3039
Serotonergic transcriptional networks and potential importance to mental health
Abstract
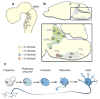
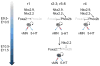
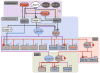
Transcription regulatory networks governing the genesis, maturation and maintenance of vertebrate brain serotonin (5-HT) neurons determine the level of serotonergic gene expression and signaling throughout an animal's lifespan. Recent studies suggest that alterations in these networks can cause behavioral and physiological pathogenesis in mice. Here, we synthesize findings from vertebrate loss-of-function and gain-of-function studies to build a new model of the transcriptional regulatory networks that specify 5-HT neurons during fetal life, integrate them into CNS circuitry in early postnatal life and maintain them in adulthood. We then describe findings from animal and human genetic studies that support possible alterations in the activity of serotonergic regulatory networks in the etiology of mental illness. We conclude with a discussion of the potential utility of our model, as an experimentally well-defined molecular pathway, to predict and interpret the biological effect of genetic variation that may be discovered in the orthologous human network.
Figures

References
-
- Gaddum JH. Ciba Foundation Symposium on Hypertension. Little, Brown and Co; 1954. Drugs antagonistic to 5-hydroxytryptamine; pp. 75–77.
-
- Muller CP, Jacobs BL, editors. Handbook of the Behavioral Neurobiology of Serotonin. Academic Press; 2010.
-
- Ishimura K, et al. Quantitative analysis of the distribution of serotonin-immunoreactive cell bodies in the mouse brain. Neurosci Lett. 1988;91:265–270. - PubMed
-
- Baker KG, et al. Cytoarchitecture of serotonin-synthesizing neurons in the pontine tegmentum of the human brain. Synapse. 1991;7:301–320. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

