Discoidin domain receptor tyrosine kinases: new players in cancer progression
- PMID: 22366781
- PMCID: PMC3351584
- DOI: 10.1007/s10555-012-9346-z
Discoidin domain receptor tyrosine kinases: new players in cancer progression
Abstract
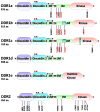
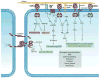
Almost all human cancers display dysregulated expression and/or function of one or more receptor tyrosine kinases (RTKs). The strong causative association between altered RTK function and cancer progression has been translated into novel therapeutic strategies that target these cell surface receptors in cancer. Yet, the full spectrum of RTKs that may alter the oncogenic process is not completely understood. Accumulating evidence suggests that a unique set of RTKs known as the discoidin domain receptors (DDRs) play a key role in cancer progression by regulating the interactions of tumor cells with their surrounding collagen matrix. The DDRs are the only RTKs that specifically bind to and are activated by collagen. DDRs control cell and tissue homeostasis by acting as collagen sensors, transducing signals that regulate cell polarity, tissue morphogenesis, and cell differentiation. In cancer, DDRs are hijacked by tumor cells to disrupt normal cell-matrix communication and initiate pro-migratory and pro-invasive programs. Importantly, several cancer types exhibit DDR mutations, which are thought to alter receptor function and contribute to cancer progression. Other evidence suggests that the actions of DDRs in cancer are complex, either promoting or suppressing tumor cell behavior in a DDR type/isoform specific- and context-dependent manner. Thus, there is still a considerable gap in our knowledge of DDR actions in cancer tissues. This review summarizes and discusses the current knowledge on DDR expression and function in cancer. It is hoped that this effort will encourage more research into these poorly understood but unique RTKs, which have the potential of becoming novel therapeutic targets in cancer.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

References
-
- Di Marco E, Cutuli N, Guerra L, Cancedda R, De Luca M. Molecular cloning of trkE, a novel trk-related putative tyrosine kinase receptor isolated from normal human keratinocytes and widely expressed by normal human tissues. The Journal of biological chemistry. 1993;268(32):24290–24295. - PubMed
-
- Zerlin M, Julius MA, Goldfarb M. NEP: a novel receptor-like tyrosine kinase expressed in proliferating neuroepithelia. Oncogene. 1993;8(10):2731–2739. - PubMed
-
- Karn T, Holtrich U, Brauninger A, Bohme B, Wolf G, Rubsamen-Waigmann H, et al. Structure, expression and chromosomal mapping of TKT from man and mouse: a new subclass of receptor tyrosine kinases with a factor VIII-like domain. Oncogene. 1993;8(12):3433–3440. - PubMed
-
- Perez JL, Shen X, Finkernagel S, Sciorra L, Jenkins NA, Gilbert DJ, et al. Identification and chromosomal mapping of a receptor tyrosine kinase with a putative phospholipid binding sequence in its ectodomain. Oncogene. 1994;9(1):211–219. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

