Correlates of spreading depolarization in human scalp electroencephalography
- PMID: 22366798
- PMCID: PMC3286336
- DOI: 10.1093/brain/aws010
Correlates of spreading depolarization in human scalp electroencephalography
Abstract
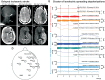
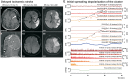
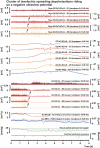
It has been known for decades that suppression of spontaneous scalp electroencephalographic activity occurs during ischaemia. Trend analysis for such suppression was found useful for intraoperative monitoring during carotid endarterectomy, or as a screening tool to detect delayed cerebral ischaemia after aneurismal subarachnoid haemorrhage. Nevertheless, pathogenesis of such suppression of activity has remained unclear. In five patients with aneurismal subarachnoid haemorrhage and four patients with decompressive hemicraniectomy after malignant hemispheric stroke due to middle cerebral artery occlusion, we here performed simultaneously full-band direct and alternating current electroencephalography at the scalp and direct and alternating current electrocorticography at the cortical surface. After subarachnoid haemorrhage, 275 slow potential changes, identifying spreading depolarizations, were recorded electrocorticographically over 694 h. Visual inspection of time-compressed scalp electroencephalography identified 193 (70.2%) slow potential changes [amplitude: -272 (-174, -375) µV (median quartiles), duration: 5.4 (4.0, 7.1) min, electrocorticography-electroencephalography delay: 1.8 (0.8, 3.5) min]. Intervals between successive spreading depolarizations were significantly shorter for depolarizations with electroencephalographically identified slow potential change [33.0 (27.0, 76.5) versus 53.0 (28.0, 130.5) min, P = 0.009]. Electroencephalography was thus more likely to display slow potential changes of clustered than isolated spreading depolarizations. In contrast to electrocorticography, no spread of electroencephalographic slow potential changes was seen, presumably due to superposition of volume-conducted electroencephalographic signals from widespread cortical generators. In two of five patients with subarachnoid haemorrhage, serial magnetic resonance imaging revealed large delayed infarcts at the recording site, while electrocorticography showed clusters of spreading depolarizations with persistent depression of spontaneous activity. Alternating current electroencephalography similarly displayed persistent depression of spontaneous activity, and direct current electroencephalography slow potential changes riding on a shallow negative ultraslow potential. Isolated spreading depolarizations with depression of both spontaneous electrocorticographic and electroencephalographic activity displayed significantly longer intervals between successive spreading depolarizations than isolated depolarizations with only depression of electrocorticographic activity [44.0 (28.0, 132.0) min, n = 96, versus 30.0 (26.5, 51.5) min, n = 109, P = 0.001]. This suggests fusion of electroencephalographic depression periods at high depolarization frequency. No propagation of electroencephalographic depression was seen between scalp electrodes. Durations/magnitudes of isolated electroencephalographic and corresponding electrocorticographic depression periods correlated significantly. Fewer spreading depolarizations were recorded in patients with malignant hemispheric stroke but characteristics were similar to those after subarachnoid haemorrhage. In conclusion, spreading depolarizations and depressions of spontaneous activity display correlates in time-compressed human scalp direct and alternating current electroencephalography that may serve for their non-invasive detection.
Figures


References
-
- Back T, Kohno K, Hossmann KA. Cortical negative DC deflections following middle cerebral artery occlusion and KCl-induced spreading depression: effect on blood flow, tissue oxygenation, and electroencephalogram. J Cereb Blood Flow Metab. 1994;14:12–9. - PubMed
-
- Canals S, Makarova I, Lopez-Aguado L, Largo C, Ibarz JM, Herreras O. Longitudinal depolarization gradients along the somatodendritic axis of CA1 pyramidal cells: a novel feature of spreading depression. J Neurophysiol. 2005;94:943–51. - PubMed
-
- Claassen J, Hirsch LJ, Kreiter KT, Du EY, Connolly ES, Emerson RG, et al. Quantitative continuous EEG for detecting delayed cerebral ischemia in patients with poor-grade subarachnoid hemorrhage. Clin Neurophysiol. 2004;115:2699–710. - PubMed
-
- Claassen J, Mayer SA, Hirsch LJ. Continuous EEG monitoring in patients with subarachnoid hemorrhage. J Clin Neurophysiol. 2005;22:92–8. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

