Safety of recombinant human deoxyribonuclease as a rescue treatment for persistent atelectasis in newborns
- PMID: 22366825
- PMCID: PMC6086641
- DOI: 10.5144/0256-4947.2012.131
Safety of recombinant human deoxyribonuclease as a rescue treatment for persistent atelectasis in newborns
Abstract
Background and objective: Pulmonary problems are vitally important in newborns. Increased intense and mucoid secretions may lead to atelectasis, pulmonary infections, respiratory distress, prolonged mechanical ventilation or even death. The aim of this study was to evaluate the safety of recombinant human deoxyribonuclease (rhDNase) in the management of persistent atelectasis in term and preterm newborns, unresponsive to the conventional treatment.
Design and setting: Prospective study of patients admitted to a general community setting of a neonatal intensive care unit between December 2007 and December 2009.
Patients and methods: The study included 22 patients (12 premature and 10 term) who were admitted to the neonatal intensive care unit because of respiratory distress and developed atelectasis, and were unresponsive to conventional treatment. Nebulized rhDNase was administered to all patients at a dose of 1 mg/m2 twice daily for 3 days. In patients who did not respond to 3 days of treatment, endotracheal rhDNase was administered at a dose of 1 mg/m2. We assessed the clinical (respiratory rate and oxygen requirement) and radiologic responses (chest radiographic score), recurrence of atelectasis, the need for a repetitive treatment, and mortality rate.
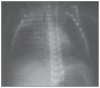
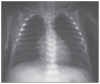
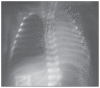
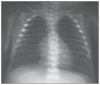
Results: A clinical and radiologic improvement of atelectasis was observed in 18 of 22 patients following 3 days of nebulized rhDNase treatment. Atelectasis relapsed in 4 patients. Following the administration of combined endotracheal and nebulized rhDNase treatment, an improvement of atelectasis was noted in all four recurrent cases. No adverse events were observed in patients because of the rhDNase treatment.
Conclusions: rhDNase treatment is a safe option and may be used as an effective method for the management of persistent atelectasis in newborns, which is resistant to other conventional treatment methods.
Figures
Similar articles
-
Efficacy and safety of nebulized recombinant human DNase as rescue treatment for persistent atelectasis in newborns: case-series.Croat Med J. 2007 Apr;48(2):234-9. Croat Med J. 2007. PMID: 17436388 Free PMC article.
-
Nebulized hypertonic saline and recombinant human DNase in the treatment of pulmonary atelectasis in newborns.Pediatr Int. 2011 Jun;53(3):328-31. doi: 10.1111/j.1442-200X.2010.03245.x. Pediatr Int. 2011. PMID: 20831650
-
Comparing the efficacy of nebulizer recombinant human DNase and hypertonic saline as monotherapy and combined treatment in the treatment of persistent atelectasis in mechanically ventilated newborns.Pediatr Int. 2012 Feb;54(1):131-6. doi: 10.1111/j.1442-200X.2011.03519.x. Pediatr Int. 2012. PMID: 22114907
-
[Recombinant human DNase in conditions other than cystic fibrosis].Ugeskr Laeger. 2010 Feb 22;172(8):616-9. Ugeskr Laeger. 2010. PMID: 20184817 Review. Danish.
-
The use of human deoxyribonuclease (rhDNase) in the management of cystic fibrosis.BioDrugs. 2005;19(3):135-44. doi: 10.2165/00063030-200519030-00001. BioDrugs. 2005. PMID: 15984899 Review.
Cited by
-
Effects of Chest Physiotherapy in Preterm Infants with Respiratory Distress Syndrome: A Systematic Review.Healthcare (Basel). 2023 Apr 11;11(8):1091. doi: 10.3390/healthcare11081091. Healthcare (Basel). 2023. PMID: 37107923 Free PMC article. Review.
-
Use of recombinant human deoxyribonuclease in pediatric intensive care unit - a single-center experience.Rev Paul Pediatr. 2021 Sep 1;40:e2020169. doi: 10.1590/1984-0462/2022/40/2020169. eCollection 2021. Rev Paul Pediatr. 2021. PMID: 34495270 Free PMC article.
-
Inhaled Pharmacotherapy for Neonates: A Narrative Review.Turk Arch Pediatr. 2022 Jan;57(1):5-17. doi: 10.5152/TurkArchPediatr.2021.21125. Turk Arch Pediatr. 2022. PMID: 35110073 Free PMC article.
-
Use of mucolytics and inhaled antibiotics in the NICU.J Perinatol. 2025 Jan;45(1):5-12. doi: 10.1038/s41372-024-02178-w. Epub 2024 Nov 19. J Perinatol. 2025. PMID: 39562833 Free PMC article. Review.
-
A Novel Maneuver to Treat Refractory Atelectasis in Mechanically Ventilated Children.J Pediatr Intensive Care. 2020 Dec 18;11(2):159-167. doi: 10.1055/s-0040-1721508. eCollection 2022 Jun. J Pediatr Intensive Care. 2020. PMID: 35734215 Free PMC article.
References
-
- Guglani L, Lakshminrusimha S, Ryan RM. Transient tachypnea of the newborn. Pediatr Rev. 2008;29:e59–65. - PubMed
-
- El Hassan NO, Chess PR, Huysman MW, Merkus PJ, de Jongste JC. Rescue use of DNase in critical lung atelectasis and mucus retention in premature neonates. Pediatrics. 2001;108:468–70. - PubMed
-
- Küpeli S, Teksam O, Dogru D, Yurdakök M. Use of recombinant human DNase in a premature infant with recurrent atelectasis. Pediatr Int. 2003;45:584–6. - PubMed
-
- Durward A, Forte V, Shemie SD. Resolution of mucus plugging and atelectasis after intratracheal rhDNase therapy in a mechanically ventilated child with refractory status asthmaticus. Crit Care Med. 2000;28:560–2. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

