Oocyte formation by mitotically active germ cells purified from ovaries of reproductive-age women
- PMID: 22366948
- PMCID: PMC3296965
- DOI: 10.1038/nm.2669
Oocyte formation by mitotically active germ cells purified from ovaries of reproductive-age women
Abstract
Germline stem cells that produce oocytes in vitro and fertilization-competent eggs in vivo have been identified in and isolated from adult mouse ovaries. Here we describe and validate a fluorescence-activated cell sorting-based protocol that can be used with adult mouse ovaries and human ovarian cortical tissue to purify rare mitotically active cells that have a gene expression profile that is consistent with primitive germ cells. Once established in vitro, these cells can be expanded for months and can spontaneously generate 35- to 50-μm oocytes, as determined by morphology, gene expression and haploid (1n) status. Injection of the human germline cells, engineered to stably express GFP, into human ovarian cortical biopsies leads to formation of follicles containing GFP-positive oocytes 1-2 weeks after xenotransplantation into immunodeficient female mice. Thus, ovaries of reproductive-age women, similar to adult mice, possess rare mitotically active germ cells that can be propagated in vitro as well as generate oocytes in vitro and in vivo.
Figures



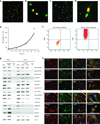
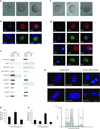
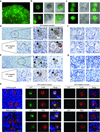
Comment in
-
The quest for human ovarian stem cells.Nat Med. 2012 Mar 6;18(3):353-4. doi: 10.1038/nm.2699. Nat Med. 2012. PMID: 22395699 No abstract available.
-
Adult human and mouse ovaries lack DDX4-expressing functional oogonial stem cells.Nat Med. 2015 Oct;21(10):1116-8. doi: 10.1038/nm.3775. Nat Med. 2015. PMID: 26444631 No abstract available.
-
Woods and Tilly reply.Nat Med. 2015 Oct;21(10):1118-21. doi: 10.1038/nm.3964. Nat Med. 2015. PMID: 26444632 Free PMC article. No abstract available.
References
-
- Zuckerman S. The number of oocytes in the mature ovary. Rec. Prog. Horm. Res. 1951;6:63–108.
-
- Johnson J, Canning J, Kaneko T, Pru JK, Tilly JL. Germline stem cells and follicular renewal in the postnatal mammalian ovary. Nature. 2004;428:145–150. - PubMed
-
- Zou K, et al. Production of offspring from a germline stem cell line derived from neonatal ovaries. Nat. Cell Biol. 2009;11:631–636. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

