NKG2D signaling on CD8⁺ T cells represses T-bet and rescues CD4-unhelped CD8⁺ T cell memory recall but not effector responses
- PMID: 22366950
- PMCID: PMC3436127
- DOI: 10.1038/nm.2683
NKG2D signaling on CD8⁺ T cells represses T-bet and rescues CD4-unhelped CD8⁺ T cell memory recall but not effector responses
Abstract
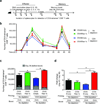
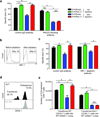
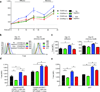
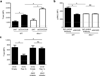
CD4-unhelped CD8(+) T cells are functionally defective T cells primed in the absence of CD4(+) T cell help. Given the co-stimulatory role of natural-killer group 2, member D protein (NKG2D) on CD8(+) T cells, we investigated its ability to rescue these immunologically impotent cells. We demonstrate that augmented co-stimulation through NKG2D during priming paradoxically rescues memory, but not effector, CD8(+) T cell responses. NKG2D-mediated rescue is characterized by reversal of elevated transcription factor T-box expressed in T cells (T-bet) expression and recovery of interleukin-2 and interferon-γ production and cytolytic responses. Rescue is abrogated in CD8(+) T cells lacking NKG2D. Augmented co-stimulation through NKG2D confers a high rate of survival to mice lacking CD4(+) T cells in a CD4-dependent influenza model and rescues HIV-specific CD8(+) T cell responses from CD4-deficient HIV-positive donors. These findings demonstrate that augmented co-stimulation through NKG2D is effective in rescuing CD4-unhelped CD8(+) T cells from their pathophysiological fate and may provide therapeutic benefits.
Conflict of interest statement
The authors have no financial conflicts of interest to disclose.
Figures

Similar articles
-
Requirement for T-bet in the aberrant differentiation of unhelped memory CD8+ T cells.J Exp Med. 2007 Sep 3;204(9):2015-21. doi: 10.1084/jem.20070841. Epub 2007 Aug 13. J Exp Med. 2007. PMID: 17698591 Free PMC article.
-
Soluble ligands for the NKG2D receptor are released during HIV-1 infection and impair NKG2D expression and cytotoxicity of NK cells.FASEB J. 2013 Jun;27(6):2440-50. doi: 10.1096/fj.12-223057. Epub 2013 Feb 8. FASEB J. 2013. PMID: 23395909
-
CD4 T cell help prevents CD8 T cell exhaustion and promotes control of Mycobacterium tuberculosis infection.Cell Rep. 2021 Sep 14;36(11):109696. doi: 10.1016/j.celrep.2021.109696. Cell Rep. 2021. PMID: 34525366 Free PMC article.
-
Release of Soluble Ligands for the Activating NKG2D Receptor: One More Immune Evasion Strategy Evolved by HIV-1 ?Curr Drug Targets. 2016;17(1):54-64. doi: 10.2174/1389450116666150630110329. Curr Drug Targets. 2016. PMID: 26122035 Review.
-
NKG2D: A versatile player in the immune system.Immunol Lett. 2017 Sep;189:48-53. doi: 10.1016/j.imlet.2017.04.006. Epub 2017 Apr 13. Immunol Lett. 2017. PMID: 28414183 Review.
Cited by
-
Murine CMV Expressing the High Affinity NKG2D Ligand MULT-1: A Model for the Development of Cytomegalovirus-Based Vaccines.Front Immunol. 2018 May 7;9:991. doi: 10.3389/fimmu.2018.00991. eCollection 2018. Front Immunol. 2018. PMID: 29867968 Free PMC article.
-
Non-oncogenic Acute Viral Infections Disrupt Anti-cancer Responses and Lead to Accelerated Cancer-Specific Host Death.Cell Rep. 2016 Oct 18;17(4):957-965. doi: 10.1016/j.celrep.2016.09.068. Cell Rep. 2016. PMID: 27760326 Free PMC article.
-
Memory CD8 T Cells Generated by Cytomegalovirus Vaccine Vector Expressing NKG2D Ligand Have Effector-Like Phenotype and Distinct Functional Features.Front Immunol. 2021 Jun 3;12:681380. doi: 10.3389/fimmu.2021.681380. eCollection 2021. Front Immunol. 2021. PMID: 34168650 Free PMC article.
-
Pro-inflammatory chemokine CCL2 (MCP-1) promotes healing in diabetic wounds by restoring the macrophage response.PLoS One. 2014 Mar 11;9(3):e91574. doi: 10.1371/journal.pone.0091574. eCollection 2014. PLoS One. 2014. PMID: 24618995 Free PMC article.
-
The multifaceted role of CD4(+) T cells in CD8(+) T cell memory.Nat Rev Immunol. 2016 Feb;16(2):102-11. doi: 10.1038/nri.2015.10. Epub 2016 Jan 19. Nat Rev Immunol. 2016. PMID: 26781939 Free PMC article. Review.
References
-
- Prlic M, Williams MA, Bevan MJ. Requirements for CD8 T-cell priming, memory generation and maintenance. Curr Opin Immunol. 2007;19:315–319. - PubMed
-
- Bourgeois C, Rocha B, Tanchot C. A role for CD40 expression on CD8+ T cells in the generation of CD8+ T cell memory. Science. 2002;297:2060–2063. - PubMed
-
- Shedlock DJ, Shen H. Requirement for CD4 T cell help in generating functional CD8 T cell memory. Science. 2003;300:337–339. - PubMed
-
- Janssen EM, et al. CD4+ T cells are required for secondary expansion and memory in CD8+ T lymphocytes. Nature. 2003;421:852–856. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

